Explore topic-wise InterviewSolutions in Current Affairs.
This section includes 7 InterviewSolutions, each offering curated multiple-choice questions to sharpen your Current Affairs knowledge and support exam preparation. Choose a topic below to get started.
| 1. |
Which of the following phenomenon is an effect acid rain ? |
|
Answer» An increase in frequency of floods |
|
| 2. |
Which of the following can be used to reduce suspended particulate matter in atmosphere in mine areas ? |
|
Answer» NaCI |
|
| 3. |
What are noble gases ? Mention their uses. |
|
Answer» SOLUTION :Noble gases are those whichare whichareinert. There arx six noble gases which are helium, neon,argon krypon, xenon and radon . `{:(,"Elements",,"Uses"),(1.,"Helium",,"Filling weather OBSERVATION BALLOONS"),(2.,"Neon",,"ADVERTISING sign boards"),(3.,"Argon, kryton and xenon",,"Filling up electric bulbs"),(4.,"Radon",,"Cancer treatment"):}` |
|
| 4. |
What will be the formula of the sulphate and sulphite of a trivalent metal that isM^(+3) ? |
|
Answer» `M_(2)(SO_(4))_(3)` 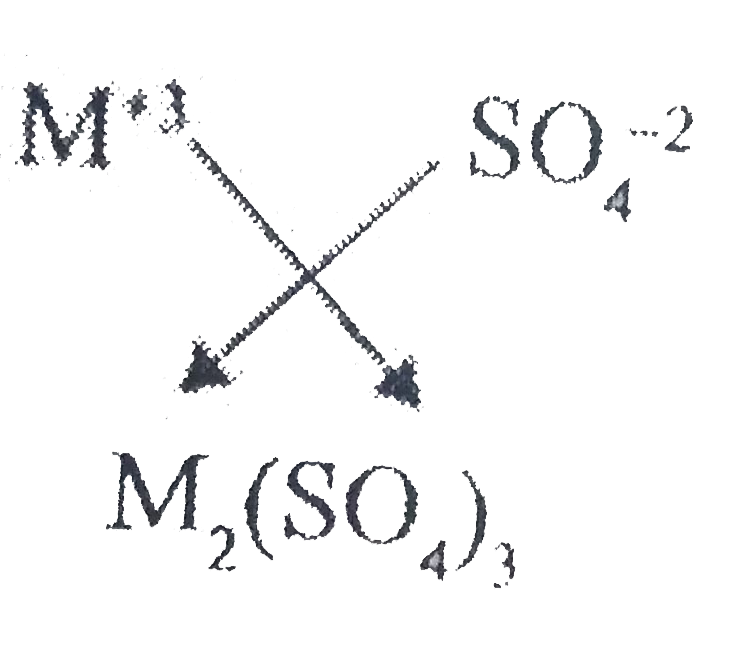
|
|
| 5. |
Which among the following is an element ? |
|
Answer» Calcium oxide |
|
| 6. |
Xenon and krypton is used in electric bulbs to slow down the sublimation of tungsten. |
|
Answer» REASON : XENON and kryptons are used to SLOW down the sublimation of tungsten in bulb. |
|
| 7. |
Whichof the following substances does not form a curdy precipitate when it is added to hard water ? |
|
Answer» POTASSIUM SALT of fatty acid. |
|
| 8. |
Why are droplets of water observed on the walls of a glass tumbler containing ice ? |
| Answer» SOLUTION :Ice present in GLASS tumbler starts MELTING by absorbing heat from the AIR AROUND the air around the glass. As a result, moisture in the air gets condensed and forms tiny water droplets on the outer walls of the container. | |
| 9. |
What are homogeneous and heterogeneous mixtures ? Give one example for each. |
| Answer» Solution :The mixture in which different CONSTITUENTS are UNIFORMLY distributed throughout the mixture are called HOMOGENEOUS mistures. Example, a mixture of alcohol and water. The MIXTURES in which different constituents are non-uniformly distributed throughout the mixture are called heterogeneous mixture. Example a mixture of sulphur and water. | |
| 10. |
Which among the following is true regarding aqueous solution of sulphur trioxide and sodium oxide? |
|
Answer» Both solutions truns BLUE litmus to red. |
|
| 11. |
While explaining modern concept of atom, Jack asked the teacher " why are electrons , assumed to be in continuous motion around the nucleus insteadof being stationary" ?Whatcould bethe explanation given by the teacher? |
| Answer» SOLUTION :The electrons are negatively CHARGED particles . THENUCLEUS being POSITIVELY charged exterts force of attraction on the electrons . Inorder to counterbalance these forces of attaraction , the electron should be in continous motion around the nucleus. | |
| 12. |
Which of the following is a true statement regarding mixtures? |
|
Answer» They have VARIABLE COMPOSITION |
|
| 13. |
Two friends Neela and Leela went to a shop, brought mango and lemon pickle of 1kg each respectively. Neeela stored the pickle in tin coated iron vessel whereas Leela stored her pickle in glass vessel. After few days, Neela found that her pickle got spoiled wheras Leela's pickle did not. Explain the reason behind it. |
| Answer» Solution :The organic acids present in pickles REACT with iron or tin metals to form HARMFUL salts. Furthermore, they corrode the CONTAINER. GLASS is inert towards the organic acids and PICKLE did not get spoiled. | |
| 14. |
Which among the following gases present in atmophere does not contribute to global warming ? |
|
Answer» NITROGEN oxides LIKE `N_(2)O` |
|
| 15. |
Which of the following phenomena is an effect of acid rain ? |
|
Answer» An increase in frequency of floods |
|
| 16. |
Water glass is used for making the lenses of telescopes. |
|
Answer» SOLUTION :False Reason : OPTICAL glass is USED for MAKING thelenses of TELESCOPES. |
|
| 17. |
Write theformulae of glucose, sugar and starch. |
| Answer» Solution :Glucose `(C_(6)H_(12)O_(6))" sugar "(C_(12)H_(22)O_(11))" and starch "(C_(6)H_(10)O_(5)_(N))`. | |
| 18. |
Which of the following can be said to provide instant energy? |
|
Answer» A solution of GLUCOSE |
|
| 19. |
Which of the following metals cannot be used in printed circuit boards? |
|
Answer» Copper |
|
| 20. |
Two metals A and B possess the same numberof electrons in L shell and they differ by 1 unit in the number of electrons present in valence shell and numberof valence electrons are greater in B than A. The ions formedget the configuration of the nearest inert gases namely argon and neon respectively. Give the probable electronic configurations ofmetals corresponding to A and B and give the formulae of their corresponding nitrite , nitrate , sulphite and bisulphite. |
|
Answer» Solution :`{:("Shell",A,B),(L,8,8),("Electronic configuration","2,8,8,1","2,8,2"),("Metal",K,Mg),("FORMULAE",(i)""KNO_(2),Mg(NO_(2))_(2)),(,(II)""KNO_(3),Mg(NO_(3))_(2)),(,(iii)K_(2)SO_(3),MgSO_(3)),(,(iv)KHSO_(3),Mg(HSO_(3))_(2)):}` Or `{:(,,A,B,),("Electronic",,"2,8,8,2","2,8,3",),("Configuration",,,,),("Metal",,CA,Al,),("Formulae",(i),Ca(NO_(2))_(2),Al(NO_(2))_(2),),(,(ii),Ca(NO_(3))_(2),AL(NO_(3))_(3),),(,(iii),CaSO_(3),Al_(2)(SO_(3))_(3),),(,(iv),Ca(HSO_(3))_(2),Al(HSO_(3))_(3),):}` |
|
| 21. |
Which of the following processes can be used to remove both temporary and permanent hardness? |
|
Answer» CLARK's method |
|
| 22. |
What is the advantage of distillation by using continuous water still over distillation by using Lebig's condenser? |
| Answer» Solution :The advantage of distillation by USING continuous water STILL is that it requires usage of lesser amount of fuel for boiling the water in the still as it had ALREADY been HEATED by CONDENSING steam. | |
| 23. |
The steps involved in the separation of camphor and send from mixture are given below. Arrange them in a proper sequence. (a) The wet cloth is put over the funnel and the stem is closed with cotton plug (b) The mixture of camphor and sand is taken in the china dish and inverted funnel is kept on it. (c ) the vapours are cooled and condensed to form the same solid and sand left behind in the dish (d) The mixture is heated gently where the vapours of camphor is formed |
|
Answer» bcda (ii) The WET cloth is put over the funnel and the stem is closed with cotton plug (iii) The mixture is heated gently where the vapours of camphor is formed (iv) The vapours are COOLED and condensed to FORM the same solid and sand left behind in the dish |
|
| 24. |
Which among the following has strong forces of attraction ? |
|
Answer» HYDROGEN chloride |
|
| 25. |
Which among the following is a pure substance ? |
|
Answer» Dilute SULPHURIC acid |
|
| 26. |
What is meant by depletion of water table? Explain in detail. |
| Answer» Solution :Sea WATER is the only proportion of water on the earth's surface which is saline is not suitable for consumption. Only river water and comprising 0.01% are sources of water. River water being limited and rivers DRYING up during the summer season, the dependence on under ground water is inevitable. The underground water is drawn out by using various DEVICES such as hand PUMPS and tube wells. This water is again replenished by means of seepage of water in to the ground during RAINY season. When there is an imbalance between underground water and its replenishment the level of water table may go down. This is called depletion of water table. | |
| 27. |
X is a base which is soluble in water. Then the metal present in X may be |
|
Answer» aluminium. |
|
| 28. |
Which of the following chemical substances can be used for removing permanent hardness of water by precipitation reaction? |
|
Answer» BAKING soda |
|
| 29. |
Whichamong the following is not a characteristic of a physical change? |
|
Answer» The CHANGE is temporary. |
|
| 30. |
What is meant by infiltration? Explain the role of infiltration in water management. |
| Answer» Solution :A part of the RAIN water REACHES the water bodies such as rivers and lakes. A part of the rain water seeps into the soil and reaches the bottom LAYERS. The rain water as well as some water from RIVER seeps through the soil and fills the empty spaces and cracks deep below the ground. This phenomenon is CALLED infiltration. The infiltration of water helps in the recharge of water table. | |
| 31. |
Zinc on reaction with dilute sulphuric acid liberates ______ gas. |
|
Answer» |
|
| 32. |
What are the disadvantages of hard water? Explain the various methods of removal of permanent hardness of water with diagrams. |
|
Answer» Solution :Disadvantages of hardness Due to the presence of various dissolved salts in water, hard water is not suitable for use for various purposes. (i) Not suitable for drinking. (ii) Due to very poor lather formation, it is not effective for washing, cleaning and bathing purposes. ltbr.(iii) Scale formation in boilers results in loss of heat and wastage of fuels in industries. (iv) Not effective for dyeing of fabrics due to improper fixing of dye to the fabric. Removal of temporary hardness (a) Boiling: However, when the water is subjected to boiling, these bicarbonates get converted to insoluble carbonates and can be removed by filtration. Since the hardness is removed by simple boiling, it is called temporary hardness and the water is called temporary hard water. `Ca(HCO_(3))_(2) overset(Delta)rarr CaCO_(3) + H_(2)O + CO_(2)` `Mg(HCO_(3))_(2) overset(Delta)rarr MgCO_(3) + H_(2)O + CO_(2)` Clark's method: This method involves the addition of slaked lime to water either in SOLID or in liquid form. This also results in conversion of soluble bicarbonates to insoluble carbonates. On filtration, soft water is obtained. `Ca(HCO_(3))_(2) + Ca(OH)_(2) rarr2CaCO_(3) darr + 2H_(2)O` `Mg(HCO_(3))_(2) + Ca(OH)_(2)rarrCaCO_(3)darr+MgCO_(3)darr+2H_(2)O` If water dissolves other minerals in it, such as chlorides and sulphates, they also cause hardness of water. However, these salts can not be removed by boiling. Hence, the hardness imparted by these salts is called permanent hardness. Removal of permanent hardness There are various methods adopted to remove permanent hardness. (a) Additionof washing soda (sodium carbonate): this method can remove both temporary as well as permanent hardness from water. Bicarbonate salts as well as sulphate and chloride salts undergo double decomposition reaction with sodium carbonate. In these reactions, the insoluble carbonates formed get precipitated and are removed by filtration. `Ca(HCO_(3))_(2)+Na_(2)CO_(3)rarrCaCO_(3)darr+2NaHCO_(3)` `MgSO_(4) + Na_(2)CO_(3) rarr MgCO_(3) darr+Na_(2)SO_(4)` `CaCl_(2) + Na_(2)CO_(3) rarr CaCO_(3) darr + 2NaCl` Permutit process: Hydrated sodium aluminum silicate is called permutit.`(Na_(2)Al_(2)Si_(2)O_(8).xH_(2)O)`. The sample of hard water is allowed to pass through a cylindrical tube FILLED with permutit. As the water passes through permutit, the calcium and magnesium ions of hard water are replaced by sodium ions. Sodium ions do not cause any precipitation with soap and do not hinder the formation of lather. Thus the water is said to be softened. After some time, the entire permutit contains `Ca^(+2)`and `Mg^(+2)` in place of `Na^(+)` ions. This is called exhausted permutit. This can be regenerated by SENDING sodium chloride solution through exhausted permutit. (c ) Ion exchange method: As the name indicates, this method involves exchange of hardness causing ions by other ions. In this method, the apparatus consists of two cylindrical tubes A and B known as cation exchanger and anion exchanger respectively. These ion exchangers contain porous solids which do not dissolve in water but swell in water. These solids are called ion exchange resins. They contain mobile groups or ions which can be exchanged with other ions. The resin containing groups or ions capable of exchanging cationsis called cation exchange resin and the resin containing groups or ions capable of exchanging anions is called anion exchange resin. H-cation exchanger results in exchange of `Ca^(+2)` or `Mg^(+2)` ions by `H^(+)` ions. Na-cation exchanger results in exchange of `Ca^(+2)` or `Mg^(+2)` ions by `Na^(+)` ions. Hard water is first passed through tube 'A' where all the positive ions are displaced by `H^(+)` or `Na^(+)` ions depending on the type of resin used. The water is then passed through tube 'B' where all the negative ions are displaced by `OH^(-)` ions present in anion exchange resin. Thus the water coming out of ion exchange apparatus now contains only pure water. This is also called demineralised water. When the cation exchanger and anion exchanger get depleted of `H^(+)` and `OH^(-)` ions, the ion exchange resins are regenerated by passing HCl solution and sodium carbonate solution through the tubes A and B respectively. `MgCl_(2) + 2H^(+) "Resin"rarr Mg - "Resin"+2HCl` `RNH_(3)OH+Cl^(-) rarrRNH_(3)Cl + OH^(-)` `H^(+) + OH^(-) rarrH_(2)O` 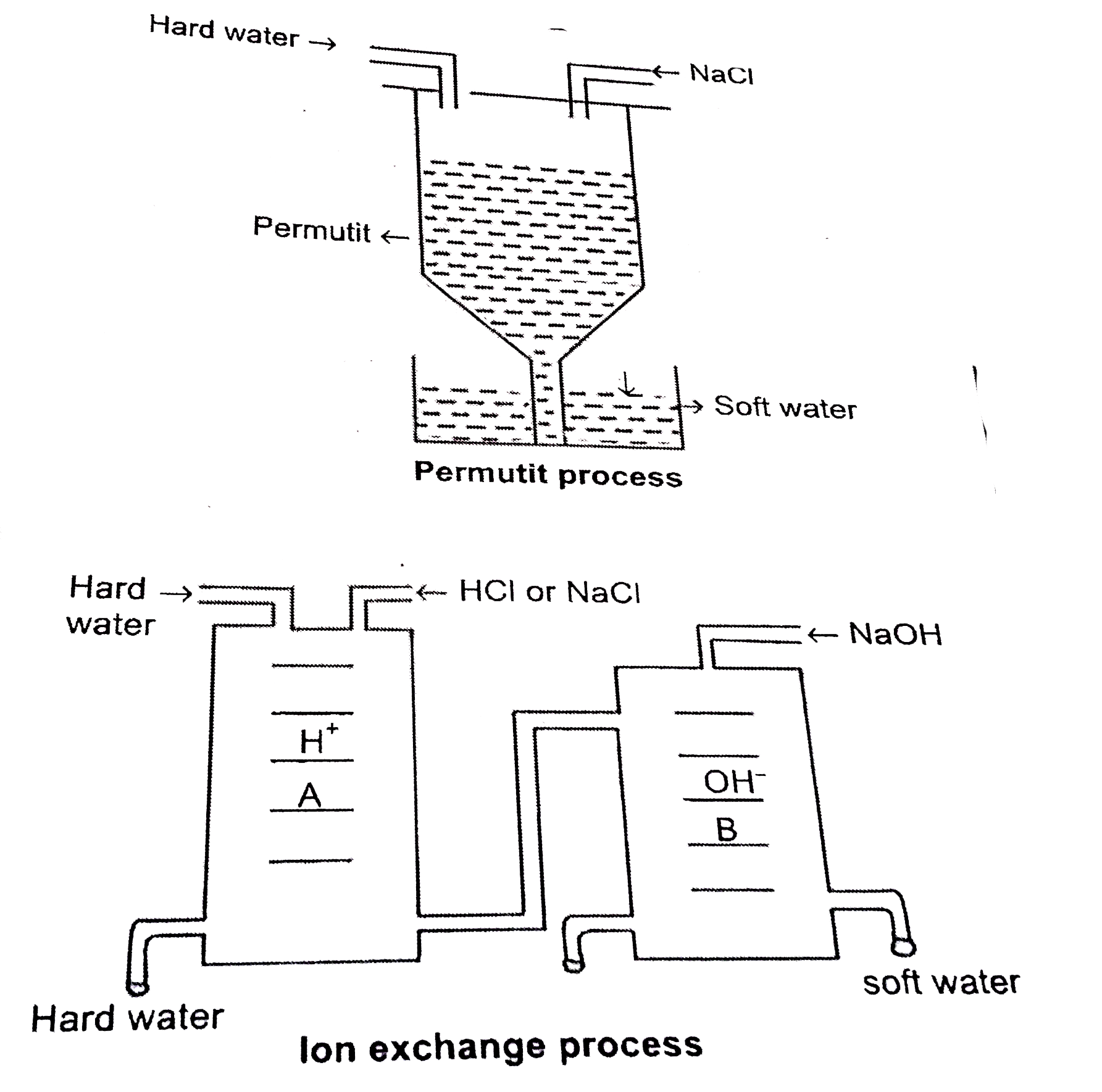
|
|
| 33. |
Which among thefollowing metals is the most suitable to be used as telecommunication cable ? |
| Answer» Solution :ELECTRICAL CONDUCTIVITY of copper is the MAXIMUM. | |
| 34. |
Two open containers A and B are filled with water and in container A, cetyl alcohol is added. Both the containers are placed at 40^(@)C. Predict the level of water in the two containers after some time and give reasons. |
| Answer» Solution :Addition of cetyl ALCOHOL reduces the rate of evaporation of WATER. Hence level of water in CONTAINER ''A'' is COMPARATIVELY higher than the level of water in container ''B''. | |
| 35. |
Which among the following reactions is not balanced ? |
|
Answer» `2KClO_(3) to2KCl+3O_(2)` `2Fe(OH)_(3)+3H_(2)SO_(4)toFe_(2)(SO_(4))_(3)+6H_(2)O` |
|
| 36. |
Watering plants by using narrow tubes is called ______. |
|
Answer» |
|
| 37. |
The solid carbon dioxide is commonly called ………. |
|
Answer» The solid carbon dioxide is COMMONLY CALLED dry ice. |
|
| 39. |
Troposphere is extended up to 6-20 km altitude from the earth's surface but the amount of oxygen is less at higher altitudes, that is on mountains or hill tops. Justify. |
| Answer» Solution :The vapour densityy of OXYGEN is 16 and that of the air is 14.4. Hence due to higher vapour density of oxygen its amount in air DECREASE on higher altitudes such as mountains that is more denser MOLECULES experience more gravitational PULL. | |
| 40. |
Which of the following is a thermosetting plastic ? |
|
Answer» POLYETHYLENE |
|
| 41. |
Vidya went to her hradfather Rajam's house to spend her summer vacation. Rajaram has coconut fields near him house. It the evening she went to the coconut field along with her grandfather. There the workers offerd her coconut water. Her grandfather told worked to provide a straw to facilitate drinking the coconut water comfortably. Can you explain the principle behind using straw for above purpose ? |
| Answer» Solution :When we SUCK ONE end of a striw dipped into the coconut water some AIR from inside the straw is drawn out. A partical vacuum is carated within the straw as a reasult coconut water is pushed up by the atmospheric pressure to fill up the vacuum and REACHES the persons mouth. Hence we can drink the coconut water. | |
| 42. |
Which chemical compound is used to reduce theacidity of the soil ? |
| Answer» Solution :GYPSUM is USED to reduce the acidity of the SOIL. | |
| 43. |
Which among the following pairs of substances has strong intermolecular forces of attraction ? |
|
Answer» Bromine, mercury |
|
| 44. |
Which among the following metal is used in allying of iron to prevent corrosion ? |
|
Answer» Zinc |
|
| 45. |
What will be the formula of the sulphate and sulphite of a trivalent metal? |
|
Answer» `M_(2)(SO_(4))_(3),M_(2)(SO_(3))_(3)` 
|
|
| 46. |
The smallest particle of an element which may or may not have independent existence is called a/an |
|
Answer» atom |
|
| 47. |
Water shows concave meniscus in a narrow glass tube. This is because. |
|
Answer» adhesive forces is STRONGER than COHESIVE force. Due to stronger adhesive forces over cohesive forces, water shows concave meniscus in narrow glass TUBE. |
|
| 48. |
Which among the following type of food can provide maximum amount of energy? |
|
Answer» Starch |
|
| 49. |
Tripositive ion of an element has 13 protons and14 neutrons . Calculate theofnumber of electrons present in K and M shells of the neutral atom. |
|
Answer» `2 :3` `{:(K,L,M),(2,8,3):}` therefore the ratio of the electrons PRESENT in K and M shell is`2:3` |
|
| 50. |
What is the valency of iron in the products formed(a) When iron is exposed to humid air for a long time?(b)When red hot iron is made to react with steam? |
|
Answer» Solution :(a) When Fe is EXPOSED to humid air for a long period of TIME , it gets rusted. `"Rust""" :""Fe_(2)O_(3).xxH_(2)O` Valency of Fe is '3' `3Fe+underset("steam")(4H_(2)O)tounderset(("ferrose ferric oxide"))(Fe_(3)O_(4)+4H_(2))` `(Fe_(3)O_(4)` is a MIXED oxide that is `FeO+Fe_(2)O_(3))` (Valency of IRON in `Fe_(3)O_(4) ` is 2 aswell 3) |
|