Explore topic-wise InterviewSolutions in Current Affairs.
This section includes 7 InterviewSolutions, each offering curated multiple-choice questions to sharpen your Current Affairs knowledge and support exam preparation. Choose a topic below to get started.
| 1. |
What is meant by synthesis reaction ? Given an example. |
|
Answer» SOLUTION :Synthesis is a combination chemical REACTION in which two ELEMENTS combine to give one SINGLE compound. Example`H_(2)+Cl_(2)to2HCl` |
|
| 2. |
What is the latent heat of fusion and latent heat of vapourization of 10 g of ice and water resepectively? |
|
Answer» 800 cal, 800 cal Latent heat of vapourization of 10 g of water is `10 xx 540` = 5400 cal |
|
| 3. |
Which of the following food items provides the maximum energy ? |
|
Answer» VEGETABLE oil |
|
| 4. |
Which of the following chemical change is a photolytic decomposition reaction? |
|
Answer» DECOMPOSITION of HOCl on standing. |
|
| 5. |
Though fat provides the maximum amount of energy, it forms a minor part ofour diet while a large part of diet contains carbohydrates? |
| Answer» Solution :Both FATS and carbohydrates are energy giving food NUTRIENTS and bothcontain CARBON, hydrogen and oxygen, but the molecularstructure of fat is more complex than that of carbohydrates. Carbohydrates breaks down to form carbon dioxide and water and water and releases energy. Combustion of fat takes a long period of time and hence carbohydrates are preferable to fats. Fats are considered as reservoirs of energywhile carbohydrates serveserve as instant SOURCE of energy. | |
| 6. |
Which among the following is used to remove moisture from the surroundings? |
|
Answer» Calcium chloride |
|
| 7. |
Which among the following pairs of elements forms cation and anion respectively ? |
|
Answer» SULPHUR and neon |
|
| 8. |
Which among the following is a detergent? |
|
Answer» SODIUM STEARATE |
|
| 9. |
Which of the following is not a chemical change? |
|
Answer» Passing of steam over redhot coke. |
|
| 10. |
What is the latent heat of fusion and latent heat of vapourisation of 10 g of ice and water respectively? |
|
Answer» 800 cal, 800 cal Latent heat of vapourisation of 10 g of steam is `10 xx 540` = 5400 cal |
|
| 11. |
What is meant by thermal decomposition ? Give an example. |
|
Answer» SOLUTION :The chemical DECOMPOSITION which TAKES PLACE in presence of heat is called thermal decomposition. Example : `2PB(NO_(3))_(2)to2PbO+4NO_(2)+O_(2)` |
|
| 12. |
What is depletion of water table? |
| Answer» Solution :When there is an imbalance between UNDERGROUND WATER and its replenishment the level of water table MAY GO down. This is called depletion of water table. | |
| 13. |
What is the difference between element and compounds ? |
|
Answer» Solution :An element is a substance which is constituted of only ONE kind of atoms and cannot be further DIVIDED by any physical or chemical means. A compound is a substance which is FORMED due to the chemical COMBINATION of TWO or more elements a definite proportion by mass. |
|
| 15. |
What are the different kinds of soap? What are the raw materials used for the manufacure of different kinds of soap? Mention their applications. |
Answer» SOLUTION :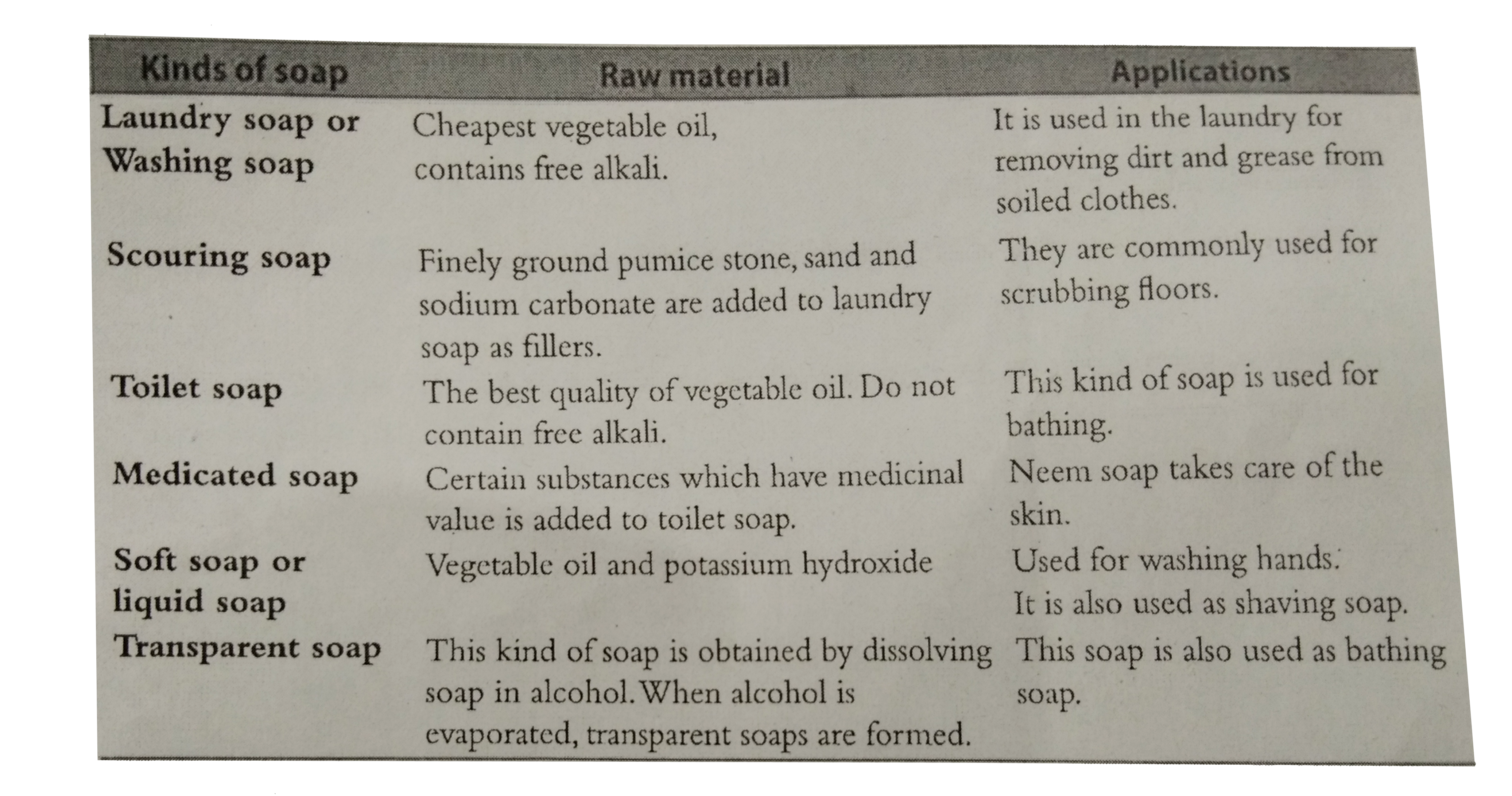
|
|
| 16. |
The salts of which among the pairs of metals cause greater water pollution? |
|
Answer» Calcium, ALUMINIUM |
|
| 17. |
What is meant by rain water harvesting? |
| Answer» Solution :UTILIZING the rian water to RECHARGE the GROUND water is called water harvesting or rain water harvesting. | |
| 18. |
What is diffusion ? Give one application. |
| Answer» Solution :The MOVEMENT of gas MOLECULES from HIGHER concentration to lower concentration is called DIFFUSION. Example : SPREADING of perfume. | |
| 19. |
Y' is obtained by the decomposition of a substance 'X'. Oxygen present in 'X' is univalent. 'Y' reacts with a neuteral oxide and evolves 'Z' which is composed of reddish brown fumes which turns moist blue litmus red. Identify 'X', 'Y' and 'Z'. |
|
Answer» `H_(2)O_(2),H_(2)" and "CO_(2)` `underset((Y))(NO)+O_(2)tounderset((Z))underset(("reddish brown"))(NO_(2))` `O_(2)`(Y) is NEUTRAL towards litmus while `NO_(2)` is ACIDIC and HENCE turns MOIST bule to red |
|
| 20. |
Which of thefollowing fertilizers replenishes only one essential among nitrogen, phosphorus |
|
Answer» Potassium NITRATE |
|
| 21. |
What are fertilizers? How are they classified ? Explain the classification of fertilizers with an example. |
|
Answer» Solution :Fertilizers are compounds or mixtures of compounds which are added to the soil in order to provide theessential nutrients to the PLANTS. Fertilizers can be categorized into two TYPE depending on the source from which they are obtained. (i) natural fertilizer and(ii) artificial fertilizer. Natural fertilizer : Example ofnatural fertilizer is manure. The excreta of live stock and human being is mixed with straw and then the mixture is buried deep into the pits for few months. The mixture decomposes there and manure is obtained. The manure is RICH in the nutrients required for the growth of the plants. Artificial fertilizer : Artificial fertilizers are the chemical compounds manufactured in the industries. They CONTAIN one or more essential nutrients required for thegrowth of the plant. Artificial fertilizers can further be classified depending on their chemical composition i.e. theessential nutrient (s) they contian. (i) Nitrogenous fertilizers : These fertilizers replenish the deficiency of nitrogen. Calcium ammoniumnitrate, ammonium SULPHATE, basic calciumnitrate and urea aer the examples of nitrogenous fertilizers. (ii) Phosphatic fertilizers : These fertilizers replenish the deficiency of phosphorus. Trianmmonium phosphate and superhosphate of lime are the examples of phosphatic fertilizers. (iii) Potash fertilizers : These fertilizers replenish the deficiency of potassium. Potassium nitrate, potassium sulphate and potassium chloride are the examples of potash fertilizers. |
|
| 22. |
Which among the following metals is the most suitable to be used as telecommunication cable? |
| Answer» Solution :ELECTRICAL CONDUCTIVITY of copper is the MAXIMUM. | |
| 23. |
Whichof thefollowing steps is not involved in the preparation of sodium chloride from sea water ? |
|
Answer» EVAPORATION |
|
| 24. |
Which of the following chemicals leads to water pollution? |
|
Answer» Fertilizers |
|
| 25. |
Which of the following pair is a major contributor to global warming ? |
|
Answer» Carbon dioxide, nitrogen oxide |
|
| 26. |
Which of the following elements is used for the purification of surface water to make it free from germs so that it can be used for drinking purpose? |
|
Answer» Oxygen |
|
| 27. |
While a teacher was her class about air and oxygen she emphasized the importance of nitrogen in the atmosphere. A student named Vivek asked the teacher-"What would happen if the percentages of nitrogen and oxygen were interchanged in the atmosphere?" The teacher praised him for the question and explained it. What was the teacher's explanation ? |
| Answer» Solution :Increased levels of oxygen increases the RATE of RESPIRATION which WOULD affect the human SYSTEM. | |
| 28. |
What do youmean bygrouting? Where does this technique find application ? |
| Answer» SOLUTION :Grouting is a TECHNIQUE used in construction activities . Pure cement or cement mixed with sand and WATER in definite proportion is applied in thegaps of construciton MATERIAL like bricks, the tiles etc., for making the surface smooth and strengthening the STRUCTURE. | |
| 29. |
Which of the following types of water does not contain dissolved gases such as oxygen and carbon dioxide? |
|
Answer» Potable water |
|
| 30. |
Which of the following water does not contain dissolved gases such as oxygen and carbon dioxide? |
|
Answer» POTABLE water |
|
| 31. |
Two salts "X" and "Y" are taken in two test tubes "A" and "B" respectively and subjected to heating Water is added to two test tubes, in case of "A" salt regains its original colour and in case of "B" water starts boiling. Then X and Y may be respectively |
|
Answer» Blue VITRIOL and limestone `underset("Blue")(CuSO_(4)*5H_(2)O overset("heating") rarr underset("Colourless")(CuSO_(4)) overset(+H_(2)O) rarr underset("blue")(CuSO_(4)*5H_(2)O)` Y is limestone, `CaCO_(3)` which on heating gives CaO, It is an exothermic reaction and hence water starts boiling. |
|
| 32. |
Which the increase in altitude, the atmopheric pressure |
|
Answer» INCREASES |
|
| 33. |
Write the symbols of the following elements.(a)Cadmium(b) Bismuth(c )Silicon(d) Argon |
|
Answer» Solution :(a) CADMIUM `to CD` (b)BISMUTH `to BI` (c ) Silicon `to Si` (d)Argon`to AR` |
|
| 34. |
Whatis the valence shell ? What is the maximum number of electrons it can have in an atom? |
| Answer» SOLUTION :The OUTERMOST shell is called valence shell and it can never have more than 8 ELECTRONS in any ATOM. | |
| 35. |
Which among the following pairs of substances has strongest intermolecular forces of attraction ? |
|
Answer» Bromine, mercury |
|
| 36. |
Which of the following metals cannot be used in printed circuit boards ? |
|
Answer» Copper |
|
| 37. |
When Rita opened the perfume bottle in the bedroom without the permission of her mother, how did her mother came to know while watching TV in the drawing room ? |
| Answer» Solution :Gas particles move with high speeds and the INTERMOLECULAR spaces in gases are very large. These properties of gases allow a gas to diffuse easily into another. As a result, the SMELL of perfume which is in vapour STATE reaces QUICKLY to the other corner of the ROOM. | |
| 38. |
When the water gets converted to ice, its volume |
|
Answer» decrease |
|
| 39. |
Two non-metallic oxides A and B are produced by burning of fossil fuels. A pollutes both air and water. B does not cause water pollution and its presence in water is essential. It causes air pollution. Identify A and B. |
|
Answer» `NO_(2),SO_(2)` |
|
| 40. |
Which among the following is an antacid pair? |
|
Answer» `Mg(OH)_(2), NAOH` |
|
| 41. |
Why are people advised not to sleep in a compound Z. 'Z' which is used for construction of monuments gets affected by acid rain. The compound X is used in while washing. Identify X, Y and Z and and write the corresponding balanced chemical equations. |
| Answer» Solution :It is DANGEROUS to sleep in closed room with COKE burning in improperly desi9gned CHULHA's because coalundergoes incomplete combustion and PRODUCES and produces carbon monoxide which is a harmful GAS and leads to respiratory problems. | |
| 42. |
Which of the following pairs is a major contributor to global warming ? |
|
Answer» Carbon DIOXIDE, oxides of nitrogen |
|
| 43. |
Which among the following pairs of bases does not decompose on heating? |
|
Answer» Calcium HYDROXIDE, potassium hydroxide |
|
| 44. |
The temperature at which solid changes to liquid is called _________ |
|
Answer» MELTING POINT |
|
| 45. |
The solvent water is used in the car radiators. Which of the following properties of water is exploited? |
|
Answer» HIGH solubility |
|
| 46. |
Which among the following pairs possess low melting points ? |
|
Answer» MEGNESIUM, mercury |
|
| 47. |
Y is obtaned by the decomposition of a substance X. Oxygen present in. Y reacts with a neutral oxide and evolves Z which is composed of reddish brown fumes which turns moist blue litmus red. Identify X, Y and Z. |
|
Answer» `H_(2)O_(2),H_(2)" and "CO_(2)` `NO+underset((Y))(O_(2))tounderset(("reddish brown"))underset((Z))(NO_(2))` `O_(2)` (Y) is neutral towards litmus whlile `NO_(2)` is acidic and HENCE turns moist BLUE to RED. |
|
| 48. |
Which of the following is the application of the highest specific heat capacity of water? |
|
Answer» Cooling of SOFTDRINK BOTTLES in ice |
|
| 49. |
What is meant by neutralisation? Give balanced equations showing neutralisation reactions of the following. (a) Slaked lime and sulphuric acid (b) Sodium bicarbonate and hydrochloric acid. (c) Magnesium carbonate and sulpuric acid. |
|
Answer» Solution :All acids react with alkalies to form SALT and water. The reaction of an acid with a base to form salt and water as the products is called NEUTRALIZATION. (i) `Ca(OH)_(2) + H_(2)SO_(4) rarr CaSO_(4) + 2H_(2)O` (ii) `NaHCO_(3) + HC1 rarr NaC1 + CO_(2) + H_(2)O` (iii) `MgCO_(3) + H_(2)SO_(4) rarr MgSO_(4) + CO_(2) + H_(2)O` |
|