Saved Bookmarks
| 1. |
Discuss the electrochemical theory of rusting. |
|
Answer» Solution :The rusting of iron can be explained on the basis of electrochemical theory which involves oxidation and reduction reaction. ACCORDING to this theory, it is believed that non-uniform surface of metal or impurities present in iron behaves like small electric cells (called CORROSION couples) in the presence of water containing dissolved oxygen or carbon dioxide. A film of moisture with dissolved `CO_(2)` acts as electrolytic solution covering the metal surface at various places. In these small electrolytic cells, pure ion acts as anode while cathodes are impure portions. The overall rusting involves the following steps : Oxidation occurs at the anodes of each electrochemical cell. Therefore, at each anode iron is oxidised to `Fe^(2+)` ions. At anode `Fe(s)toFe^(2+)(aq)+2e^(-)""......(i)` hus, the metal atoms in the lattice pass into the solution as ions, leaving electrons on the metal itself. These electrons move towards the cathode region through the metal. At the cathode of each cell, the electrons are taken up by hydrogen ions (reduction takes place). The `H^(+)` ions are obtained either from water or from acidic substances in water : `H_(2)OhArrH^(+)+OH^(-)""......(II)` or `CO_(2)+H_(2)OtoH^(+)HCO_(3)^(-)""......(iii)` At cathode : `H^(+)+e^(-)toH""......(iv)` The hydrogen atoms on the iron surface reduce dissolved oxygen. `4H+O_(2)to2H_(2)O""......(v)` Therefore, the overall reaction at cathode of different electrochemical cells may be WRITTEN as `4H^(+)(aq)+O_(2)(g)+4e^(-)to2H_(2)O(l)""......(vi)` The overall redox reaction may be written by multiplying reaction at anode, Eq. (i) by 2 and adding reaction at cathode Eq. (iv) to equalise number of electrons lost and gained, i.e., Oxidation half reaction `Fe(s)toFe^(2+)(aq)+2e]xx2` Reduction half reaction `4H^(+)(aq)+O_(2)(g)+4e^(-)to2H_(2)O(l)` Overall cell reaction `2Fe^(2+)+4H^(+)(aq)+O_(2)(g)to2Fe^(2+)(aq)+2H_(2)O(l)` The ferrous ions are oxidized further by atmospheric oxygen to `Fe^(2+)` (as `Fe_(2)O_(3)` and form rust `4Fe^(2+)+O_(2)(g)+4H_(2)Oto2Fe_(2)O_(3)+8H^(+)` and `underset("Rust")(Fe_(2)O_(3)+xH_(2)O)to2Fe_(2)O_(3).xH_(2)O` The `H^(+)` ions PRODUCED above are also used for reaction (iv). `4Fe^(2+)+O_(2)+4H_(2)Oto2Fe_(2)O_(3)+8H^(+)(aq)` `Fe_(2)O_(3)+xH_(2)OtoFe_(2)O_(3).xH_(2)O` 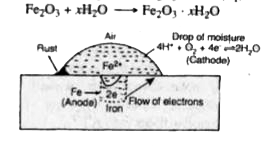
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?