Saved Bookmarks
| 1. |
Discuss the nature of bonding in the following coordination entities on the basis of Valence Bond Theory : [CoF_(6)]^(3-) |
|
Answer» Solution :`[CoF_(6)]^(3-)` -- `sp^(3)d^(2)`, octahedral, paramagnetic. FORMATION of `[CoF_(6)]^(3-)` Oxidation state of Co is +3. Electronic configuration of `Co^(3+)` No pairing of electrons `sp^(3)d^(2)` hybridisation 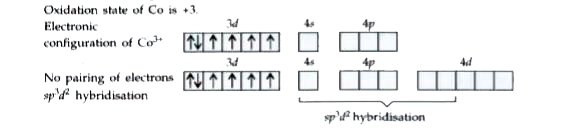 As `F^(-)` ION is a weak ligand, pairing of electrons does not TAKE place, 4s, 4p and two of the 4d orbitals hybridise (`sp^(3)d^(2)` hybridisation) giving rise to octahedral complex. As the d orbitals of the next shell are INVOLVED in hybridisation, it is an outer complex. As there are four unpaired electrons, the complex ion is paramagnetic. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?