Saved Bookmarks
| 1. |
Explain abnormal molar masses. Also explain association and dissociation of solute. |
|
Answer» Solution :Dissociation : when ionic COMPOUNDS when dissolved in water dissociate into cations and anions, and increases number of soluble particles in solution which is known as dissociation. For example, if we dissolve one mole of KCl in water, we expect one mole each of `K^(+)` and `Cl^(-)` ions to be released in the solution. If we ignore interionic attractions, one mole of KCl in one kg of water would be expected to increase the boiling point by `2xx0.52 K = 1.04 K`. Now if we did not know about the degree of dissociation, we could be led to conclude that mass of one mole of KCl would be 37.25 g. This brings into light the rule that, when there is dissociation of solute into ions, the EXPERIMENTALLY determined molar mass is always lower than the true value. 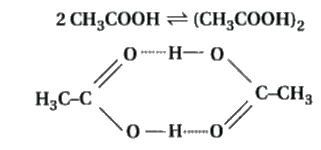 Molecules of acetic acid dimerise in benzene due to hydrogen bonding. This NORMALLY happens in solvents of low dielectric constant. In this case the number of particles is reduced due to dimerisation. Association of molecules is depicted as follows : It can be undoubtedly stated here that if all the molecules of ethanoic acid associate in benzene, then `Delta T_(B)` or `Delta T_(f)` for ethanoic acid will be half of the normal value. The molar mass calculated on the basis of this `Delta T_(b)` or `Delta T_(f)` will, therefore, be twice the expected value. Such a molar mass that is WITHER lower or higher than the expected or normal value is called as abnormal molar mass. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?