Saved Bookmarks
| 1. |
Explain IUPAC nomenclature of Co-ordination compounds with suitable examples. |
|
Answer» Solution :IUPAC nomenclature : The formula of a compound is an abreviated description of the CONSTITUTION of the compound. The following rules are prescribed by IUPAC for naming of Co-ordination compounds. i) Positive ions are named first followed by negative ions. eg. : Potassium hexacyanoferrate (II), `K_(4)[Fe(CN)_(6)]` i) Within the Co-ordination sphere ligands are named before the metal atom/ion. However, in formulae, metal ion is written first. eg. : Tetraammine copper (II) sulphate `[Cu(NH_(3))_(4)]SO_(4)` iii) Prefixes are used to denote the number of same ligands that the present in the Coordination sphere. Complex ions are denoted in paranthesis ( ) and prefixed by bis, tris etc. Examples :  eg. : `[Co(NH_(2)CH_(2)CH_(2)NH_(2))Cl_(2)` Cl is named as dichloro bis (ethylendeiamine) cobalt (III) chloride iv) Ligands are named in alphabetical order. eg. : `[PtCl_(2)(NH_(3))_(2)` diammine dichloro platinum (II) v) Anionic ligands are denoted by a suffix 'O' and netural ligands are denoted by their original NAMES. eg. : `Cl^(-)-" Chloro", CN^(-)-"Cyano"` Exception for the above are indicated below.  vi) Oxidation state of the metal ion is indicated by Romen numerical in parenthesis. eg. : `[AG(NH_(3))_(2)][Ag(CN)_(2)]` is named as diammine silver (I) dicyanoargentate (I). vii) If the charge of the Co-ordination entity is negative, the name of the metal ends with a suffix-ate. eg. : `[Co(SCN)_(4)]^(2-)-tetrahiocyanato cobaltate (II) Some metal ions are denoted by their names from which their symbols are derived eg. : Fe-ferrate Pb-plumbate Sn-stannate Ag- argentate Au-aurate viii) Prefixes cis - and trans are used to designate adjacent and opposite geometric locations of the ligands, in a complex. eg. : 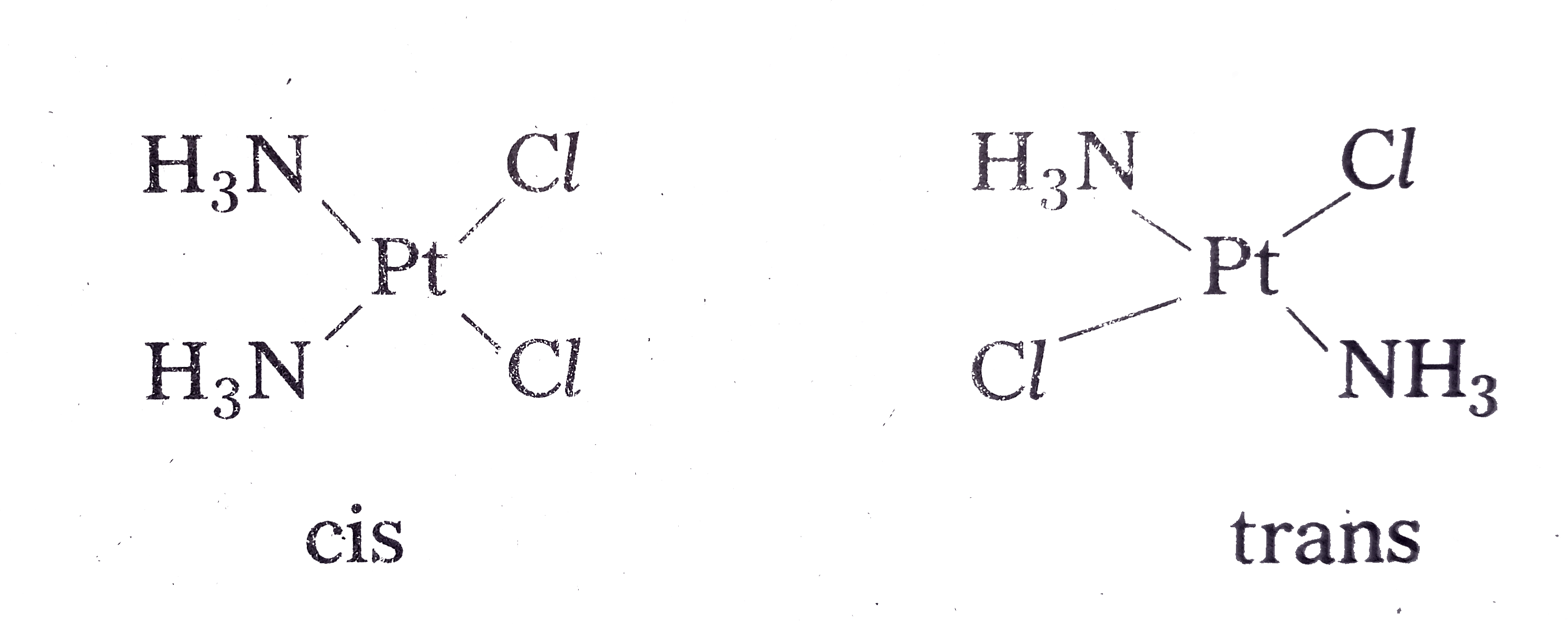 ix) Bridging ligands between two metal ions in a Co-ordination entity are denoted by prefix `mu`(greek letter 'mu'). eg. : `[(NH_(3))_(4)Co(OH)(NH_(2))Co(NH_(3))_(4)]^(+)` is named as `mu`amido-`mu` hydroxo bis (tetraammine) cobalt (IV). i) Tetrahydroxozincate (II) - `[Zn(OH)_(4)]^(-2)` ii) Hexa ammine cobalt (Ill) sulphate - `[Co(NH_(3))_(6)]_(2)(SO_(4))_(3)` iii) Potassium tetrachloropalladate - `K_(2)[PdCl_(4)]` iv) Potassium tri(oxalato) chromate (III) - `K_(3)[Cr(C_(2)O_(4))_(3)]` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?