Saved Bookmarks
| 1. |
Explain the construction and working of Westron standard cell. |
|
Answer» Solution :Westron cell `(` figure`)`consists of an `H-` shaped glass vessel with two platinum leads in its two arms at the bottom. The positive electrode is mercury covered with a paste of mercurous sulphate `(Hg_(2)SO_(4))` and `Hg`. The negative electrode consists of `Cd-Hg` amalgam containing `12%`to `24% Cd` by weight . Some crystals of `3CdSO_(4).8H_(2)O` are kept over the electrodes and the remaining portion is filled wiht a saturated solution of `CdSO_(4)`. The ends are then sealed. This is represented as `:` `Cd(Hg)|Cd^(2+)(` Saturated `)||Hg_(2)SO_(4)(s)|Hg` Westron CELLS are reversible and are not damaged by the PASSAGE of current through it. Their `EMF` does not vary much change in temperature. The reversible REACTION that occurs in the cell during its operation is `:` Anode reaction `(` from amalgam `):` `Cd(s) rarr Cd^(2+)+2e^(-)` `[E^(c-)._((Cd^(2+)|Cd))=-0.403V]` Cathode reaction `:` `Hg_(2)SO_(4)(s) +2e^(-)rarr2Hg(1)+SO_(4)^(2-)` `[E^(c-)._(Hg_(2)SO_(4)|2Hg)]=0.6153V` Cell reaction `:` `ulbar(Cd(s)+Hg_(2)SO_(4)rarr Cd^(2)=2Hg(1)+SO_(4)^(2-))` `E^(c-)._(cell)=(E^(c-)._(reduction ))_(c)-(E^(c-)._(reduction))_(a)` `=0.6153V-(-0.403)=1.0183V.` 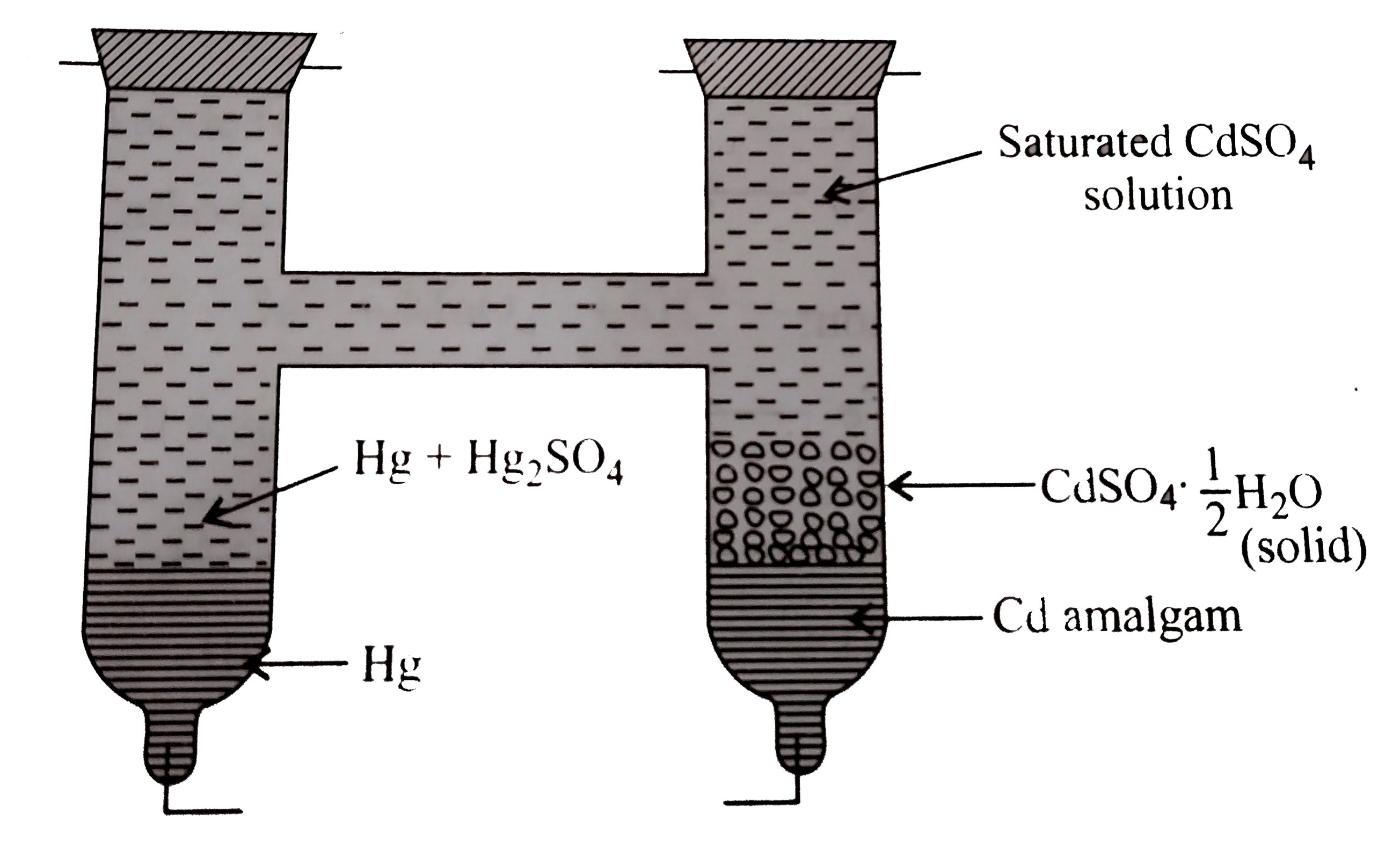 Since the activities of element and solid substance `(Cd-Hg_(2)SO_(4))` and mercury are unity and `[Cd^(2+)]` and `[SO_(4)^(2-)]` in saturated solution are constant and the standard potential of `Cd|Cd^(2+)` and the standard potential of `Hg,Hg_(2)SO_(4)|SO_(4)^(2-)` are also constant, the `E_(cell)` is constant `(=1.0183V)` at `298K`. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?