Saved Bookmarks
| 1. |
Explain the following: a) Why alkenes are more reactive than alkanes? b) Acetylene reacts with ammoniacal silver nitrate solution or ammoniacal cuprous chlroide solution or sodamide to form an acetylide while ethylene does not. c) But-2-ene shows geometrical isomerism but but-1-ene does not show. d) Why has but-1-yne a larger dipole moment (0.80 D) than but-1-ene (0.40D) ? e) Wjhuy alkylnes are slightly more soluble in water than alkenes and alkanes? f) Cyclopropane is more reactive than cyclobutane. g) Cyclopentane is more reactive than cyclobutane. h) Which isomer of C_(4)H_)(9)Br yeilds only a single alkene on dehydrobromination? i) Write the products of dehydrochlorination of the following: (j) Which of the following reactions would provide a better synthesis of 2-pentene? k) Write the major products of dehydration of l) Comparison of heat of hydrogenation for the following alkenes: m) Write the intermolecular step and give the product of the following reactioni: n) Give the structures of an optically active alkene (A) having the lowest molecular mass, which on catalyst hydrogenation gives an optically inactive compound (B). |
|
Answer» Solution :a) `pi`-bond is weaker than a greater bond and is easily broken. The `pi`-electron are less firmly bound to carbon nuclei. b) Acetylene reacts to form acetylide because it contains acidic hydrogen. c) In but-1-ene, the carbon atom linked by double bond is ATTACHED with two hydrogen atoms (similar GROUPS) and thus does not show geometrical isomerism. 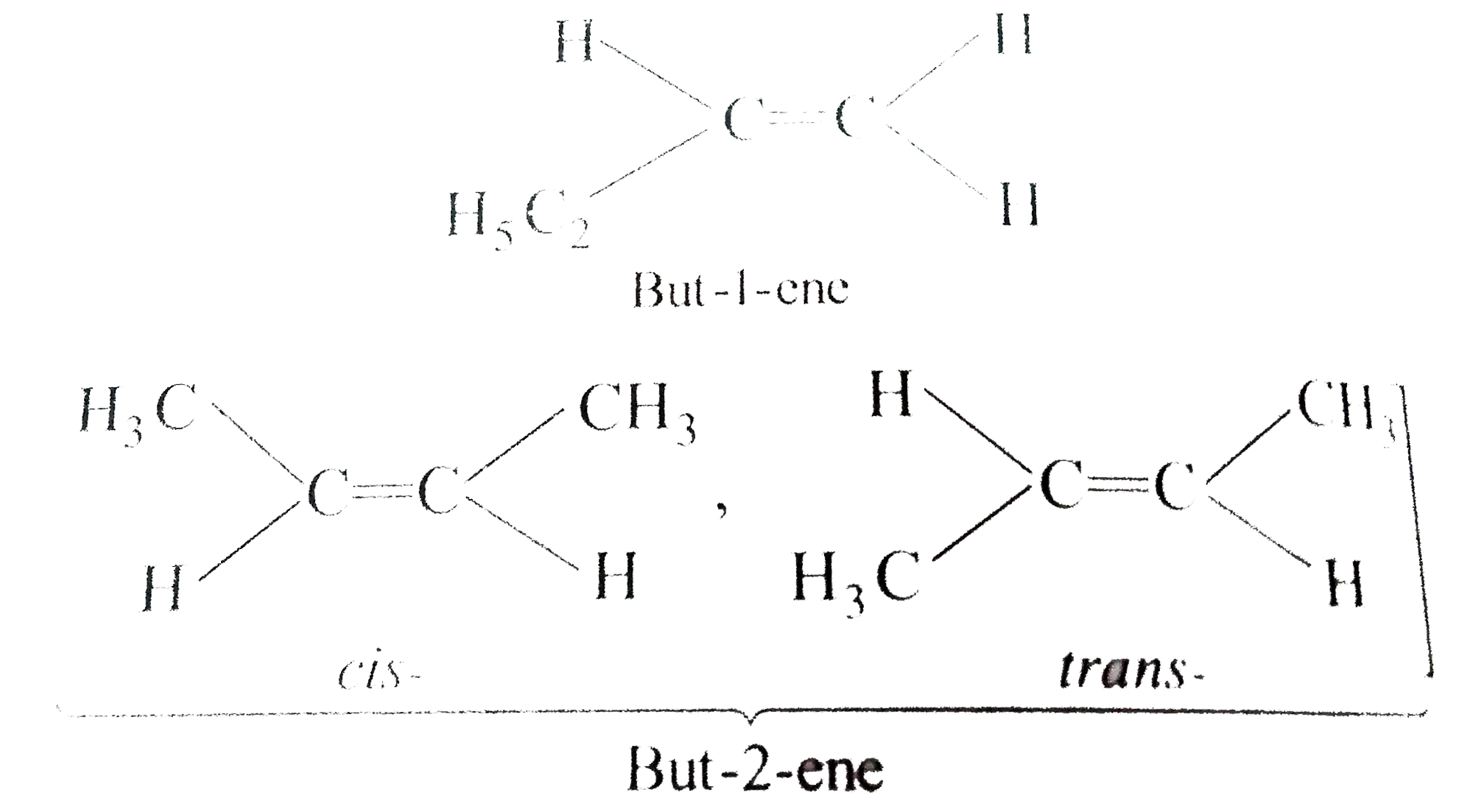 d) A `C-C_(sp)` bond is more polarised than `C-C_(sp^(2))` bond because carbon with more s-character is more electronegative. e) Alkuynes are somewhat more polar in nature and thus, their solubility is slightly more in water. h)  i) 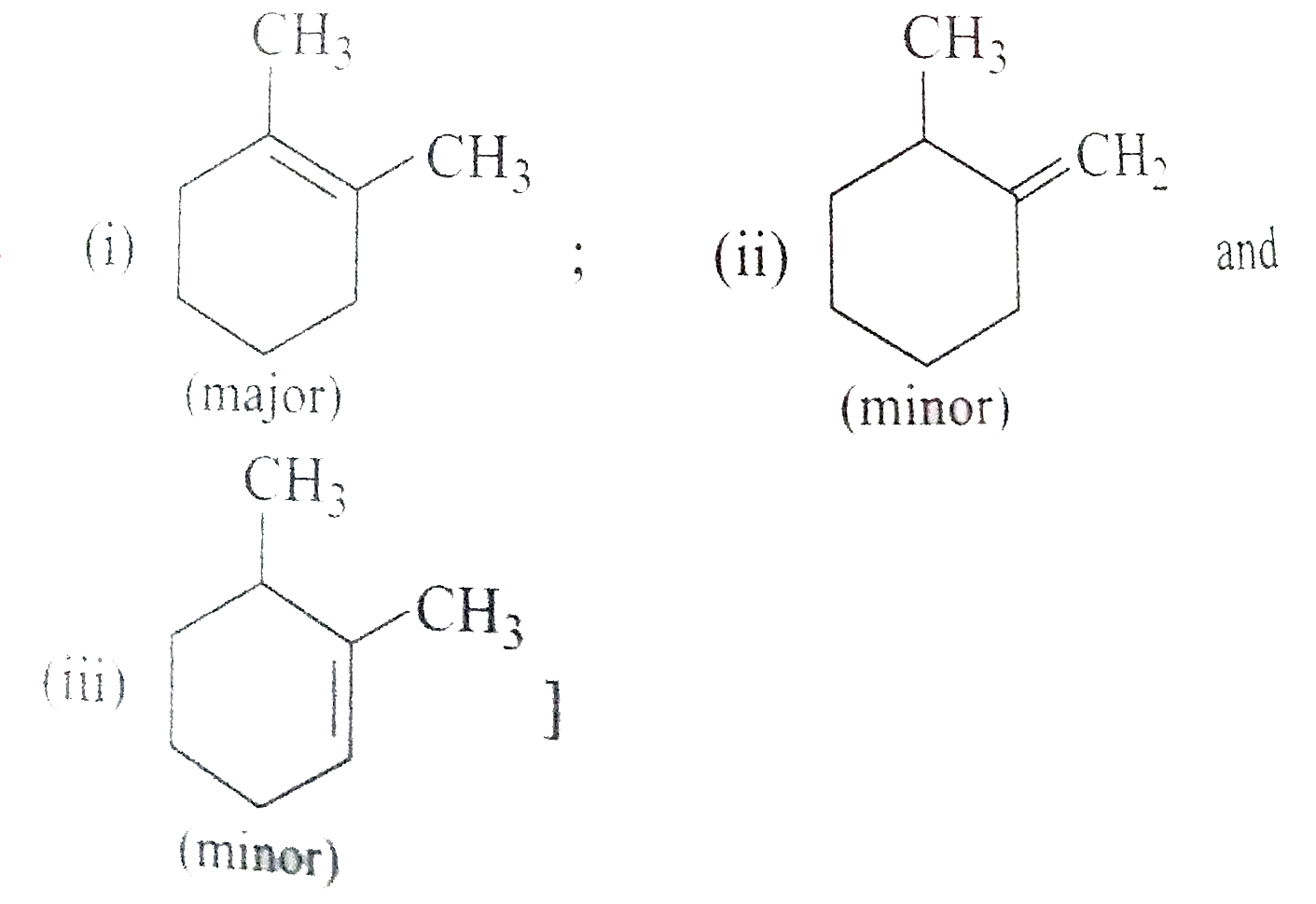 j) I) is better because it gives only one product.  While (ii) gives a mixture of  k) 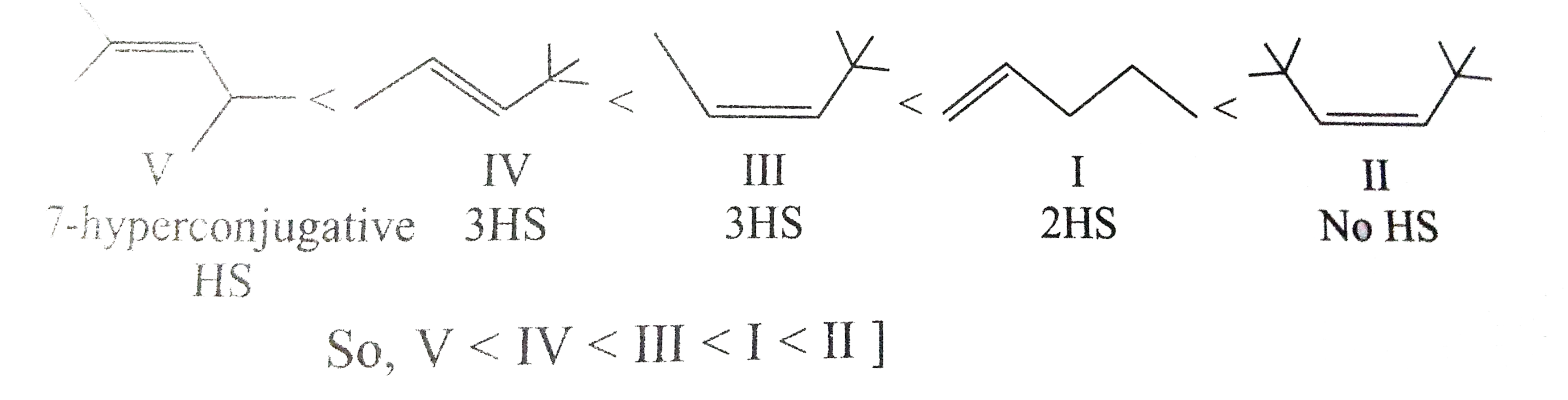 l) Greater is the stability of alkene, lower the heat of hydrogenatin. Out of cis- and trans-isomes, the trans-isomer is more STABLE than cis-isomer in which two alkyl groups lie on the same side of the double bond and hence cause STERIC hindrance, THEREFORE, heat of hydrogeneation of trans-isomer is less than that of cis-isomer.  m) 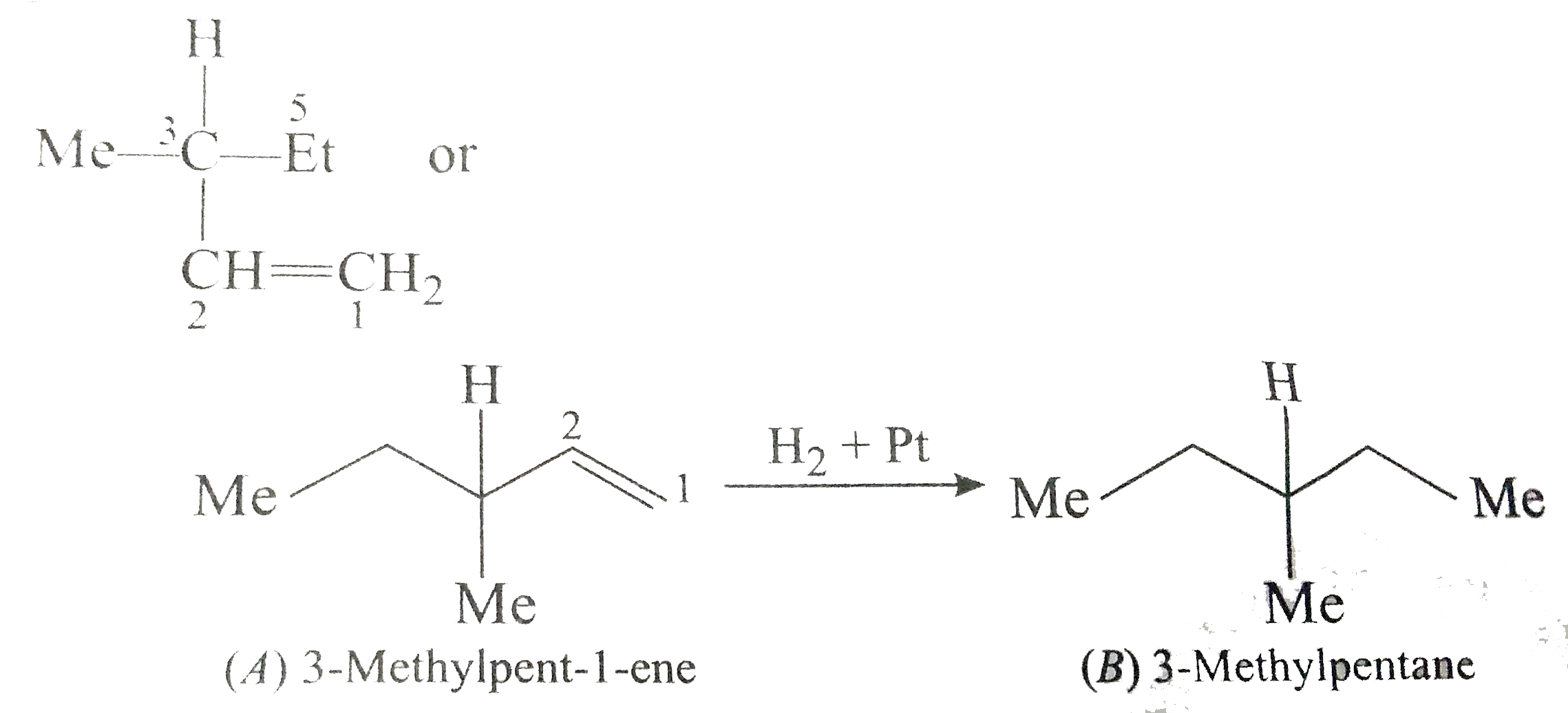
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?