Saved Bookmarks
| 1. |
Explain the following with proper reasonin (i) Why do halogen acids which are easily added to (ii) Pure HCN fails to react with aldehydes. (iii) Why only RCHO, RCOCH_(3) and cyclic ketones react with NaHSO_(3)? (iv) Chloral hydrate is a stable compound even it is a gem-diol. (v) Formation of oximes and other ammonia derivatives from carbonyl compounds require slightly acidic media for maxiumum rate (vi) In the following reaction, three isomers are present in the equilibrium which is most stable (vii) teh strurcture of two isomeric oximes formed by the reaction of acetophenon with hydroxylamine hydrochloride. (viii) Why o-hydroxy benzaldehyde is a liquid at room temperature while p-hydroxy benzaldehyde is high metling solid? |
Answer» Solution :(i) Halogen acids readily combine to the polarised 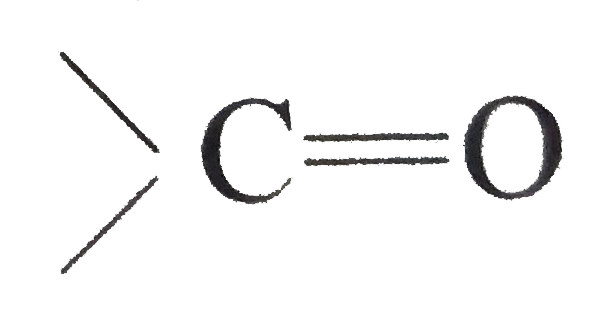 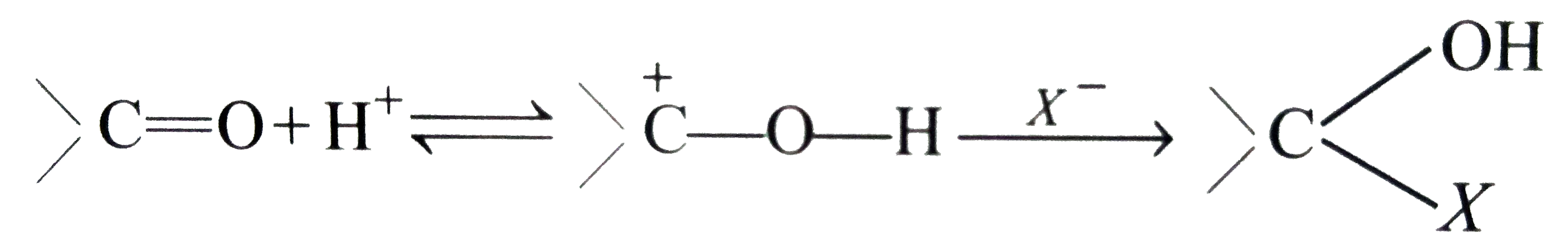 The addition product being similar to gem-dihydroxyle compound decomposes into the original substance 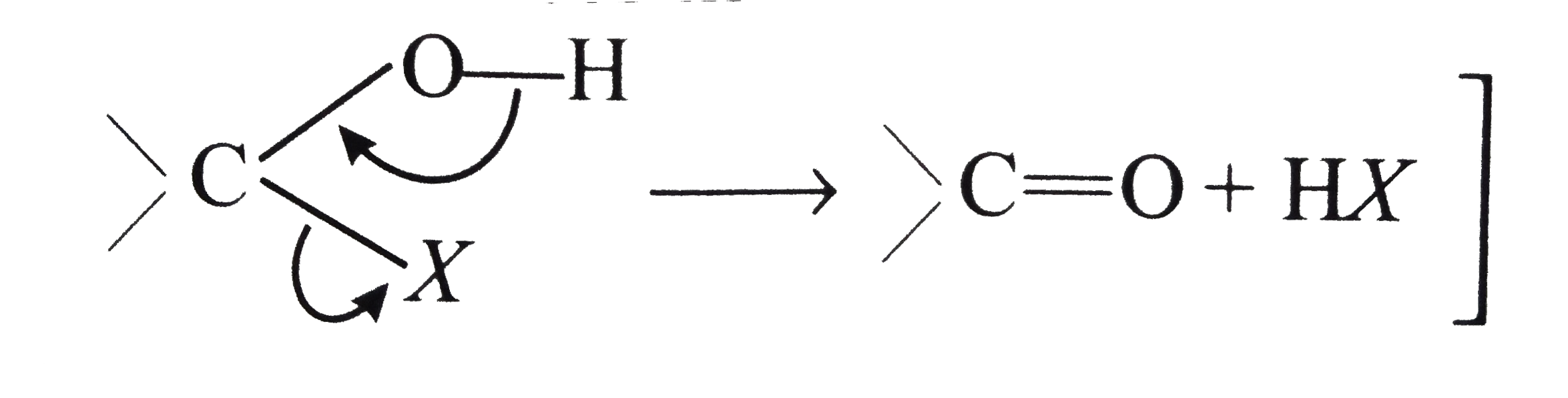 (ii) HCN is a covalent compound an does not furnish `CN^(-)` ions. However, in the presence of bases, it furnishes sufficient concentration of `CN^(-)` ions and the reactionproceeds. `HCN+OH^(-)rarrH_(2)CN^(-)` (iii)`SO_(3)^(2-)` is a large ion. Its addition is possible only under the condition that  grouping is not sterically hindered as is the case for RCHO `RCOCH(3)` and CYCLIC ketones grouping is not sterically hindered as is the case for RCHO `RCOCH(3)` and CYCLIC ketones (iv) In chloral, the -I effect of three chlorine atoms destabilise the  structure as it uts `delta^(+)` charge on the carbon adjacent to the carbonyl `overset(delta^(+))C`. In the chloral hydrate, adjacent positive charges are not present. AS a result, weak nucleophiles like water readily add to the structure as it uts `delta^(+)` charge on the carbon adjacent to the carbonyl `overset(delta^(+))C`. In the chloral hydrate, adjacent positive charges are not present. AS a result, weak nucleophiles like water readily add to the 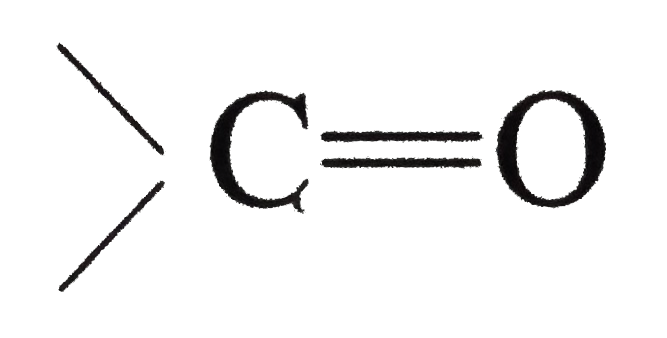 group formin chloral group formin chloral hydrate there by shifting the equilibrium almost entirely towards right. Besides this, the intramolecular H-bonding between Cl and H atom of the -OH group further stabilies the chloral hydrate molecule. The hydrogen bonding in the molecule also increases its stability 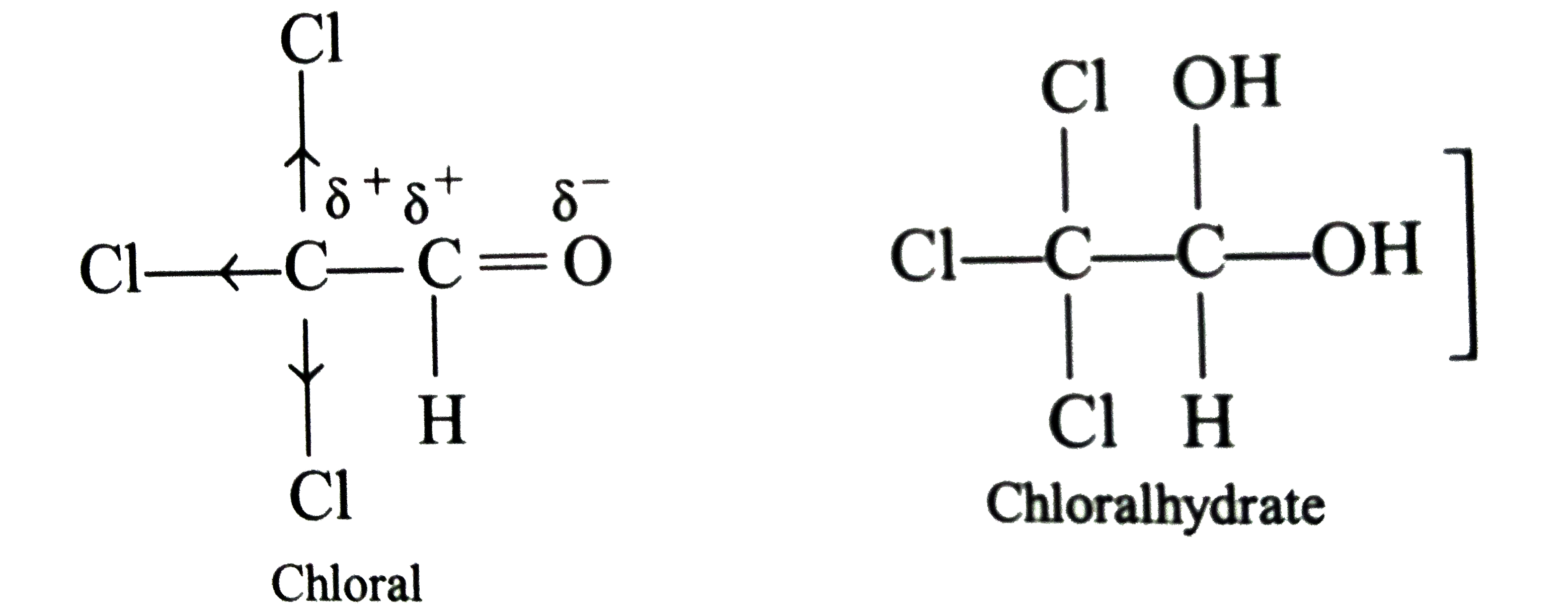 (v) In WEAKLY acidic medium, carbonyl group is protonated,  Thsi faciliates the addition of Lewis bases. However, is strongly acidic media, the nitrogen of the reagent is protonated through the unshared pair of electrons and thus the reagent ATTACK the carbonyl group. In basic, media, there is no protonation of carbonyl group. (VI) Crotonaldehyde is most stable, sicneit is a conjugatedaldehyde. (vii)  (viii) o-Hydroxy benzaldehyde involves intramolecular hydrogen bonding, hence it is a liquid because itsintermolecular forces is low. On the other hand, p-hydroxy benzaldehyde involves intramolecular hydrogen bonding and therefore, it is a soilid. 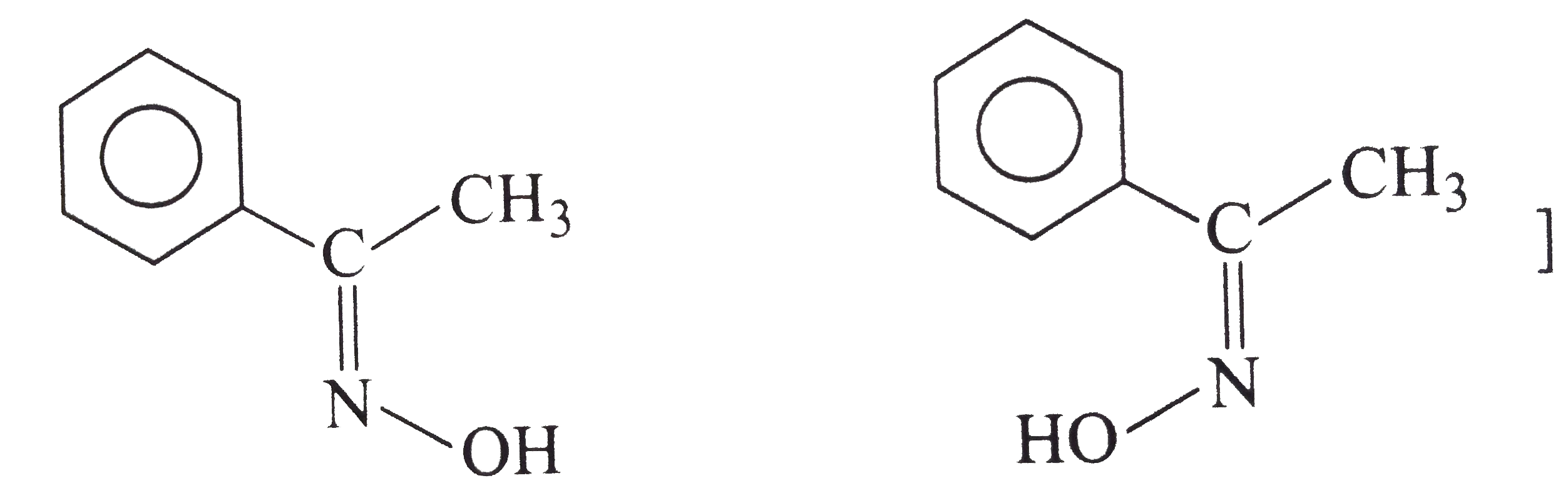 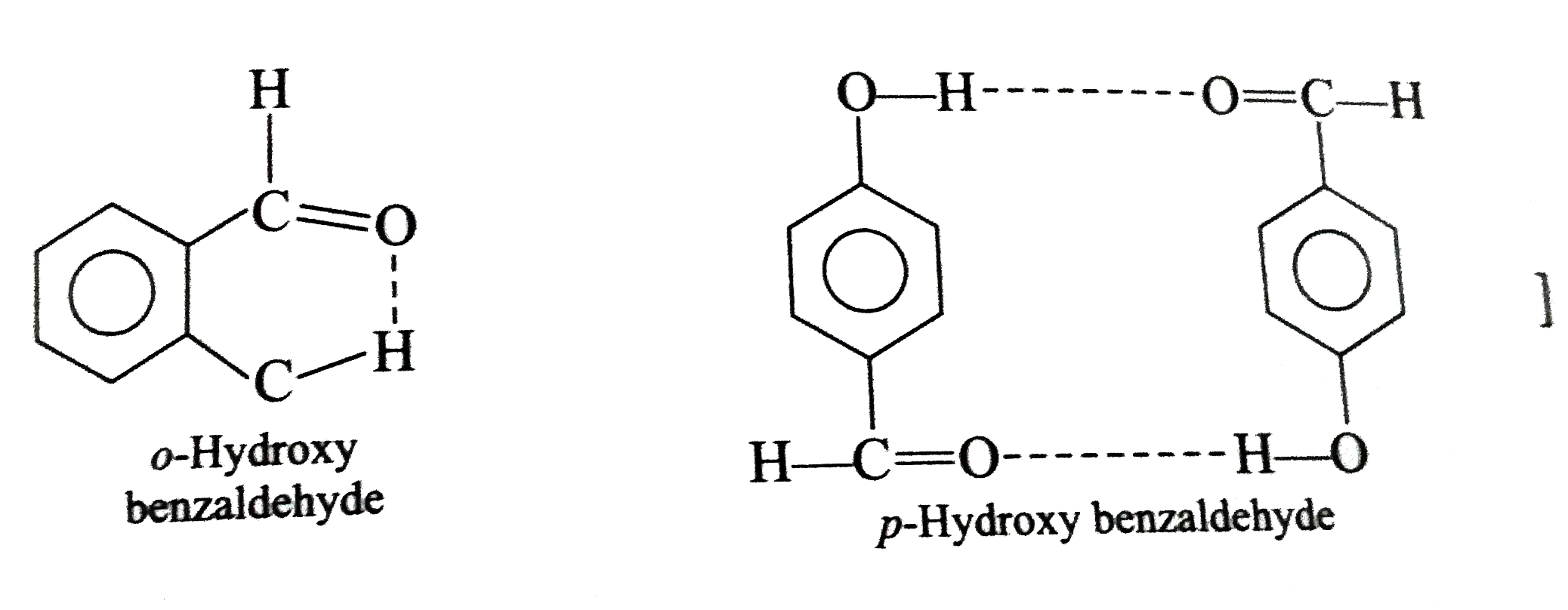
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?