Saved Bookmarks
| 1. |
Explain the following with proper reasoning: (i) Although p-hydroxy benzoic acid is less acidic thanbenzoic acid but alpha-hydroxy benzoic acid (salicylic acid) is 15 times more acidic than benzoic acid. (ii) Oxidation of toluene by acidic KMnO_(4) gives poor yield of benzoic acid while oxidation of p-O_(2)NC_(6)H_(4)CH_(3) gives good yield of p-nitrobenzoic acid. |
Answer» Solution :(i) Presence of -OH group (electron withdrawing) on BENZOIC acid decreases the acidic nature because negative charge on carboxylate ion DECREASE thereby MAKING carboxylate ion less stable. The resonance effectbeing stronger as compared to inductive effect, there is net increase in electron densifty at o-and p- positions, p-hydroxy benzoic acid is, therefore, less acidicthan benzoic acid. However, in o-hydroxy benzoic acid, there is intermolecular hydrogen bonding which STABILIZES o-hydroxy benzoate ion (conjugated base) to greater extent and thereby increasing acidic nature. 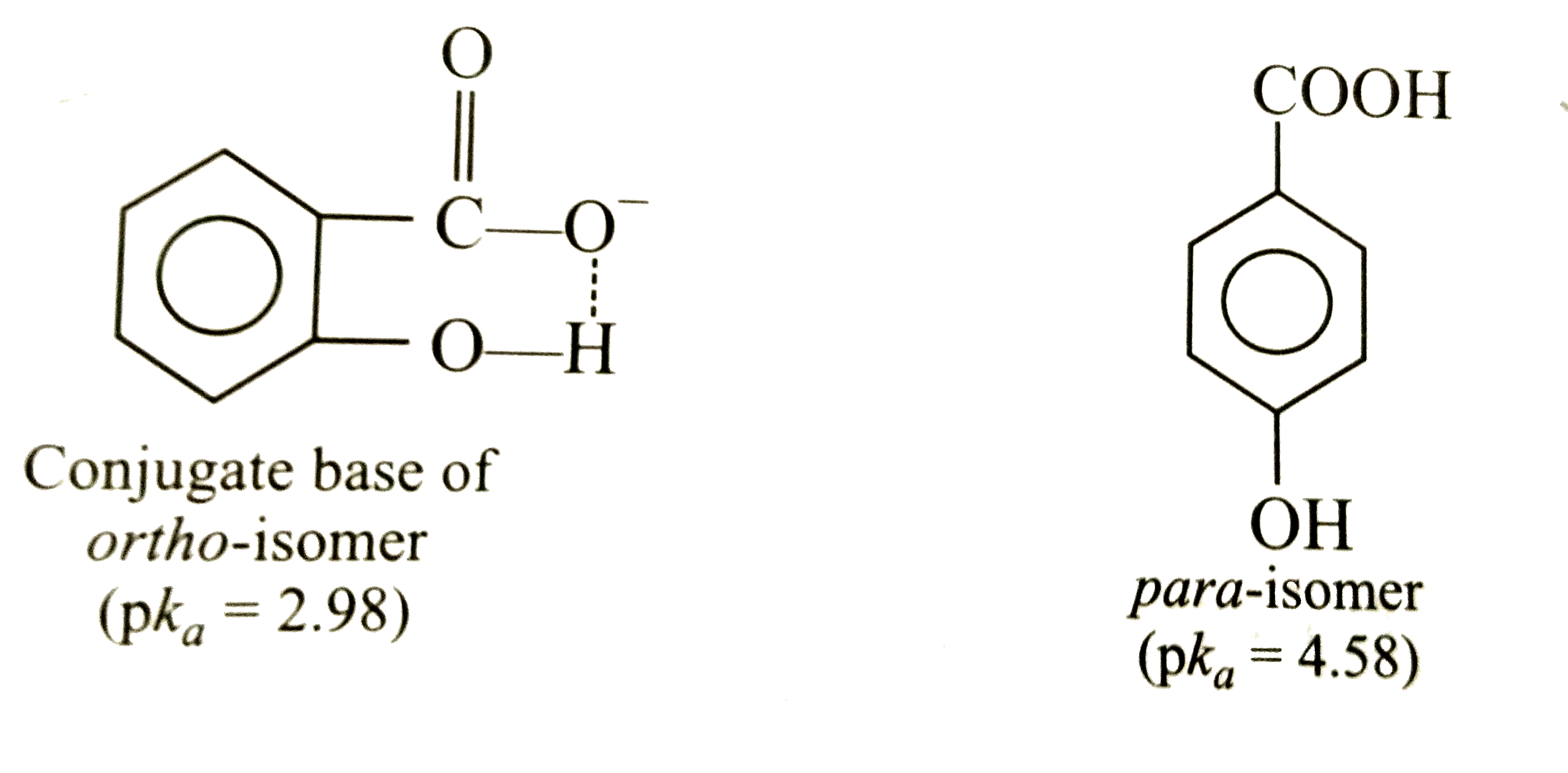 This anomalous behaviour of groups when present m ortho-position is termed ortho effect. (ii) Oxidant is an electrophile, it can DESTROY the ring in case of toluene. But in para-nitrotoluene, the `-NO_(2)~ group deactivates the benzene ring and thus increases the yield of p-nitrobenzoic acid. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?