Saved Bookmarks
| 1. |
Explain vapour pressure of solutions of solids in liquids. |
Answer» Solution :Liquids at a GIVEN temperature vapourise and under equilibrium conditions the pressure exerted by the vapours of the liquid over the liquid phase is called vapour pressure.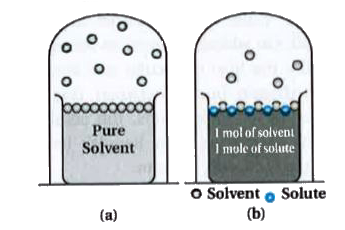 In a PURE liquid the entire surface is occupied by molecules of the liquid. If anon - volatile solute is added to a solvent to given a solution, the vapour pressure of the solution is solely from the solvent alone. This vapour pressure of the solution at a given temperature is found to be lower than the vapour pressure of the pure solvent at the same temperature. In the solution, the surface has both solute and solvent molecules, there by the fraction of the surface covered by the solvent molecules gets reduced. Consequently, the number of solvent molecules escaping from the surface is correspondingly reduced, thus, the vapour pressure is also reduced. The decrease in the vapour pressure of solvent depends on the QUANTITY of non - volatile solute present in the solution, irrespective of its nature. Let `p_(1)` be the vapour pressure of the solvent, `x_(1)` be its MOLE fraction, `p_(1)^(0)` be its vapour pressure in the pure state. Then according to Raoult.s law`p_(1)prop x_(1)` and `p_(1)=x_(1)xx p_(1)^(0)` The proportionality constant is equal to the vapour pressure of pure solvent, `p_(1)^(0)`. A plot between the vapour pressure and the mole fraction of the solvent is linear. 
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?