Saved Bookmarks
| 1. |
From the concetration of C_(4)H_(9)Cl (butyl chloride) at different times given below, calculate the average rate of reaction: C_(4)H_(9)Cl+H_(2)O rarr C_(4)H_(9)OH+HCl during different intervals of time. |{:([C_(4)H_(9)Cl] (mol L^(-1)),t (s)),(0.100,0),(0.0905,50),(0.0820,100),(0.0741,150),(0.0671,200),(0.0549,300),(0.0439,400),(0.0210,700),(0.017,800):}| |
|
Answer» Solution :First determine the difference in the concentration over difference intervals of time and thus determine the average rate by dividing `Delta[R]` by `Delta t`. Average rate of hydrolyiss of butyl CHLORIDE 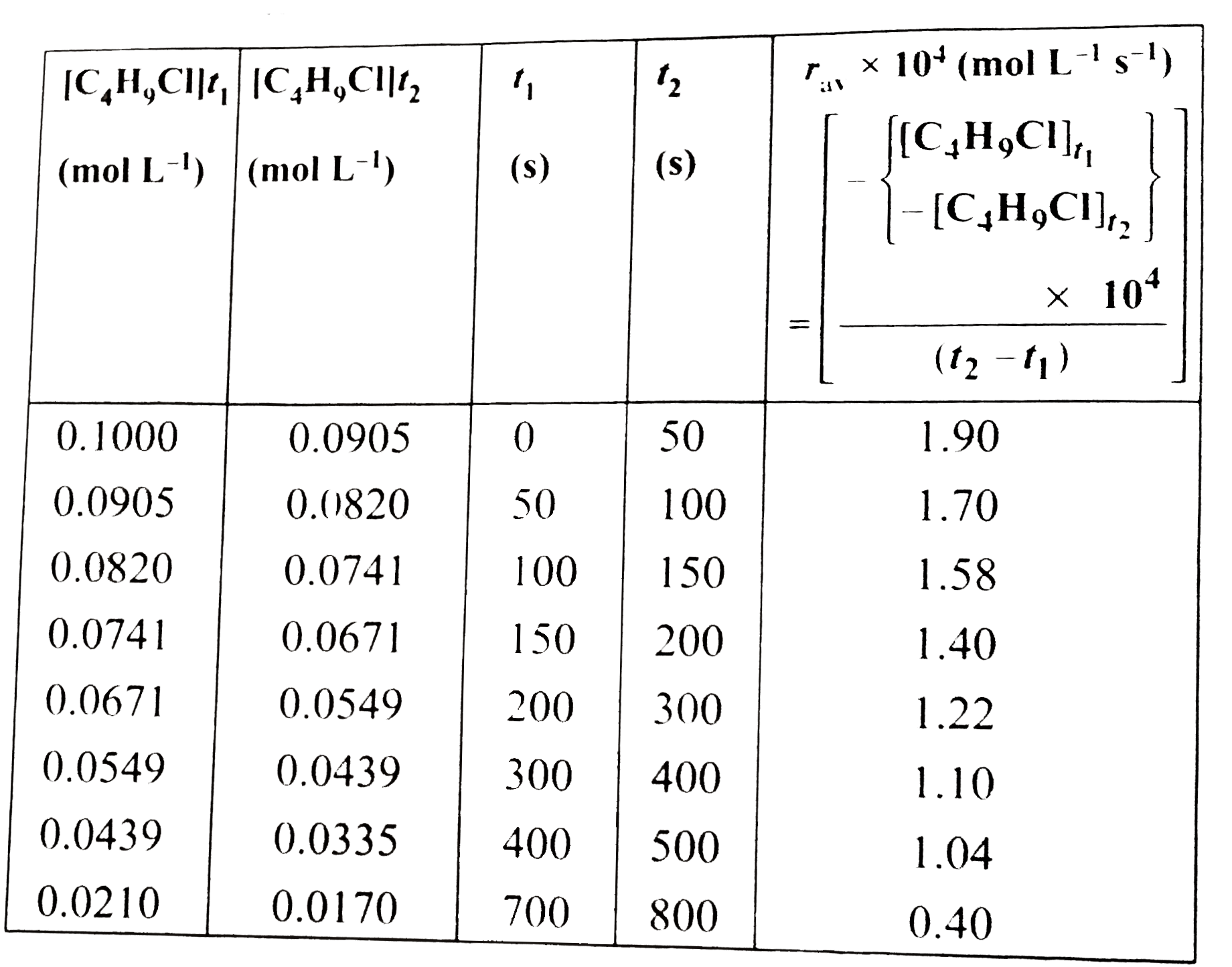 It is clear that the average rate falls form `1.90 xx 10^(-4) mol L^(-1)s^(-1)` to `0.4 xx 10^(-4) mol L^(-1) s^(-1)`. However, the average rate cannot be used to predict the rate of a reaction at a particular instant as it WOULD br constant for the time interval for which it is calculated. So to express the rate at a particular moment of time, the instantaneous rate is determine. It is obtained when we conisder the average rate at the smallest time interval say `dt` (i.e., when `Delta t` APPROACHES zero). Hence, mathematically, for an infinitesmally small `dt`, instantaneous rate is given by: `r_(av) = (-Delta[R])/(Delta t) = (Delta[P])/(Delta t)` As `t rarr 0, ot r_("inst") = (-d[R])/(dt) = (d[P])/(dt)`...(i) It can be determined graphically by drawing a tangent at time `t` on either isdes of the curves for concentration of `R` and `P` vs time `t` calculatingits slope. So in ILLUSTRATION 4.1, `r_(inst)` at `600 s`, for example, can be calculated by plotting the concentration of butyl chloride as a function of time. A tangent is drawn that touches the curve at `t = 600 s`.  The slope of this tangent gives the instantaneous rate. So,`r_("inst")` at `600 s = ((0.0165 - 0.037)/((800-400)s))mol L^(-1)` `= 5.12 xx 10^(-5) mol L^(-1) s^(-1)` At `t = 250s, r_("inst") = 1.22 xx 10^(-4) ,ol L^(-1) s^(-1)` `t = 350 s, r_("inst") = 1.0 xx 10^(-4) mol L^(-1) s^(-1)` `r = 450 s, r_("inst") = 6.4 xx 10^(-5) mol L^(-1) s^(-1)` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?