Saved Bookmarks
| 1. |
Give suitable explanation for the following: (i) Chloral hydrate is a geminaldiol but still stable. How? (ii) Out of and CH_(3)CH_(2)CHO which is more reactive nucleophilic addition? (iii) Why does pure HCN fail to reac with aldehydes and ketones? (iv) Hydrazones of aldehydes and ketones are not prepared in strongly acidic mediu. Why? (v) Sodium bisulphite is used to purify aldehydes and ketones. Explain. |
Answer» Solution :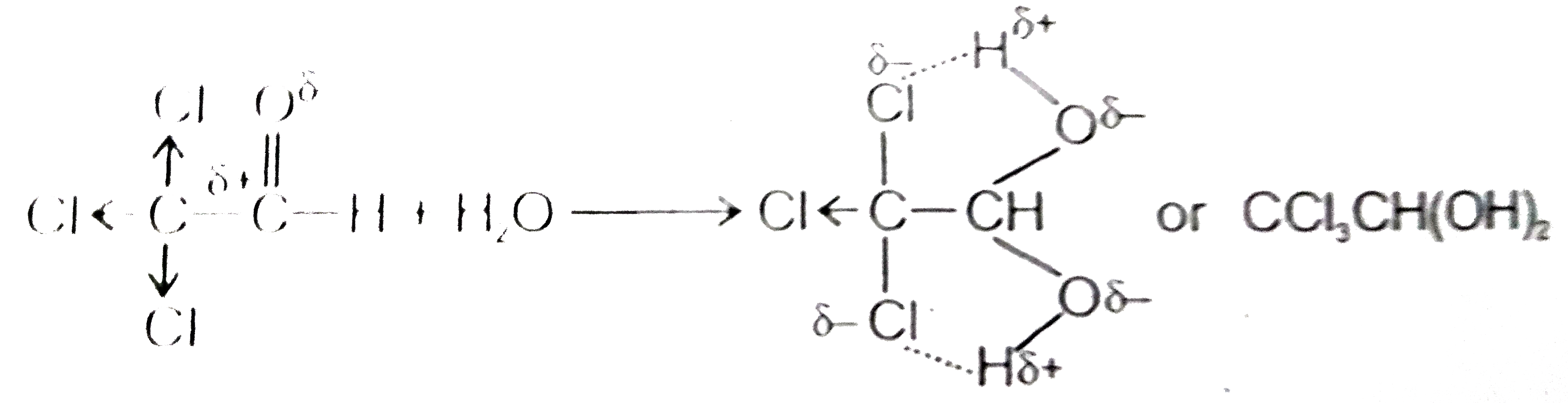 Chloral hydrate is a geminaldiol and still stable. It is due to INTRAMOLECULAR hydrogen bonding between Cl and OH groups of adjacent carbon atoms. (II) Propionaldehyde is more reactive, because in benzaldehyde, due to -M effect of -CHO group, it pulls electrons from benzene ring as a result electrophilicity on carbon of carbonyl group decreases and attack of nucleophile does not take place so efectively. (iii) HCN is a mainly a covalent compound and cannot provide `CN^(-)` ions readily for nucleophilic attack on carbonyl compounds. When reaction is carried out in the presence of base, it helps in ionising the acid and in liberating `CN^(-)` (iv) During hydrazone preparatin, hydrazine (i.e., `NH_(2)NH_(2)`) acts as a nucleophile. In stronly acidic medium hydrazine gets protonated and does not act as a nucleophile. As a result hydrazone of aldehydes and ketones cannotbe prepared. ( v) Except BENZOPHENONE, aldehydes and ketones react with saturated solution `NaHSO_(3)` to form white crystaline PRECIPITATE of thier addition products. Which can be easily decomposed by aqueous HCl or NaOH to regenerate these compounds in pure state. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?