Saved Bookmarks
| 1. |
Give the decreasing order of following with their properties as indicated. A. Decreasing basic, mucliophllic and fugacity orders: a. I, H_(2)O, II. CH_(3)OH, III. O overset(o-)(H), IV. CH_(3)O^(o-) b. I. H_(2)O, II. C_(2)H_(5) OH, III. O overset(o+)(H), IV. C_(2)H_(5)O^(o-) c. I. HCO_(3)^(o+), II. F^(o-) III. F_(3) C - COO^(o-), IV. NO_(3)^(o-) B. The decreasing order of ArSN reaction: a. I. PhCl II. p-NO_(2) - C_(6)H_(4) - Cl III. 2,4,6- Trinitro chlorobenze IV. 2.4- Dintro chlorobenzene I. PhF II. p-NO_(3)S - C_(6) H_(4) - F III. p-HOOC - C_(6) H_(4) - F IV. Ip-NO_(2) - C_(6) H_(4) - F C. I. PhCl II. p-NO_(2) - C_(6) H_(4) - Cl III. o-NO_(2) - C_(6) H_(4) - Cl IV. m-NO_(2) - C_(6)H_(4) - Cl C. The decreasing order of SE reaction: a. I. PhCl, II. C_(6) H_(6) III. PhCH_(3), IV. PhOMe b. I. PhCH_(3) II. o-MeO-C_(6) H_(4) - Me III. m-MeOC_*(6) H_(4) - Me IV. p-MeO - C_(6)H_(4) - Me c. I. PhNH_(2), II. PhNHCHOCH_(3) III. PhNHCOPh , IV. PhNHTs d. I. PhNH_(2) II. o-NP_(2) - C_(6) H_(4) - NH_(2) III. m-NUO_(2) - C_(6) H_(4) - NH_(2) IV. p-NO_(2) - C_(6) H_(4) - NH_(2) |
|
Answer» <P> Solution :a. Convert the given bases into acid by adding `H^(o+)`. Find the order of acidic character.Remember `CH_(3)OH` is a slightly acid than `H_(2)O` but other alchois are weaker acidsthan `H_(2)O`. Order of acidic character: `CH_(3) O underset(o+)(H_(2)) gt H_(3) O^(o+) gt CH_(3) OH gt H_(2)O` `pK_(n)` value: `-2.5, 1.74, 15.5, 15.74`. For basic character remove `H^(o+)` ion and reverse the acidic order. Order of basic character: `CH_(3)OH lt GH_(2)O lt CH_(3)O^(o-) lt O^(o+)H` `(II) lt (I) lt (IV) lt (III)` Decreasing order: `(III) gt (IV) gt (I) gt (II)` Nuclephillicity order: (Since the nuclephlic centre is same, teh orders of basicaly and nucleopphilicy are same.) `(III) gt (I) gt (IV) gt (III)` b. Rember `H_(2)O` is a stronger acid than `C_(2) H_(5)OH` Order of acidic cahracter: `H_(3) O^(o+) gt C_(2) H_(5) O^(o+) H_(2) gt H_(2)OOH` Order of basic character: `H_(2)O lt C_(2) H_(5)OH lt OH lt C_(2)H_(5)O` `(I) lt (II) lt (III) lt (IV)` Decreasing order: `(IV) gt (III) gt (II) gt (I)` Nuclephilic order: `(IV)(II) gt (II) gt (I)` Basically and nucleiphilcilty orders are same, due to teh same nucleopphilic centre. Fugacity order (reverse of basic order): `(I) gt (II) gt (III) gt (IV)` C. Order of acidic character: `HNO_(3) gt F_(3)C - COOH gt HF gt H_(2) CO_(3)` `pK_(4)` value: `-1.4, 0.18, 3.2, 3.7` Basic order: `NO_(3)^(o+) lt F_(3)C - COO^(o-) lt F^(o-) lt HCO_(3)^(o-)` `(IV) lt (III) gt (II) lt (I)` Decreasing basicand nucleophillic orders: `(I) gt (II) gt (III) gt (IV)` Nucleophililic order and basic orders are same. Fugacity order (reverser of basic order): `(IV) gt (III) gt (II) gt (I)` B. A. `ArSn` reactions are facoured by `EWG`ART `o-` adbn `p-` positonsmore the`EWG`presentatthesepositons, faster is the `ArSN` reaction. The decreasingorder of `ArSN` reasction: `(III) gt (IV) gt (II) gt (I)` `(III) implies` Three `(NO_(2))` groups at `o-` and `p-` (IV) implies` Two `NO_(2)` groups at `o-` and `p-`, `(II) implies ` One(NO_(2))` groups at `p-`. `(I) implies (Cl)` group` b. The decreasing oreder of `ArSN` reaction: `(II) gt (IV) gt (III) gt (I)` `EW` power by resonance of `(-SO_(3)H) gt (-NO_(2)) gt (-COOH)` in all `F` has to be substitudedby `Nu^(o-)` (althogh `-I` effect of `(-NO_(2)) gt (-SO_(3)H)` (`:'EN` of `NgtS` ) but `-R` effect of `(-SO_(3)H)` gropu than in `(-NO_(2))` and `(-COOH), -R` power is equal but `-I` effect of `(-NO_(2))` froup `o-NO_(2) gt p-NO_(2) gt m- NO_(2)` (at `o-` and `p-` positobns `-R` effect is equal, but `-1` effect at `o-` position`-R` effect is equal. but`-1` effect at `o-0` positonsis slightlygreater than that at `p-` postion and at `m-` positon only `-1` effect is operative). The decreasingorder of `ArSN` reaction: `(III) gt (II) gt (IV) gt (I)` C. `SE` reaction is favoured by `EDG`. More the `EDG` faster is the `SE` reaction. a. `ED` power of `(-OME)` (by `+R` and `-I` effect) `gt (_CH_(3))``(buy `+1` and `H.C`) gt BENZENE `gt (-Cl)` (deactivating by `-1` effect). `:.` decreasing`SE` reaction order: `(IV) gt (III) gt (II) gt (I)` b. `ED` POWEROF `(-OMe) group: `(p - OMe) gt ("orhto", - OMe) gt (-CH_(3)) gt (m 0 OMe)`  `:.` Dereasing `SE` reaction order: `(I) gt (II) gt (III) gt (IV)` 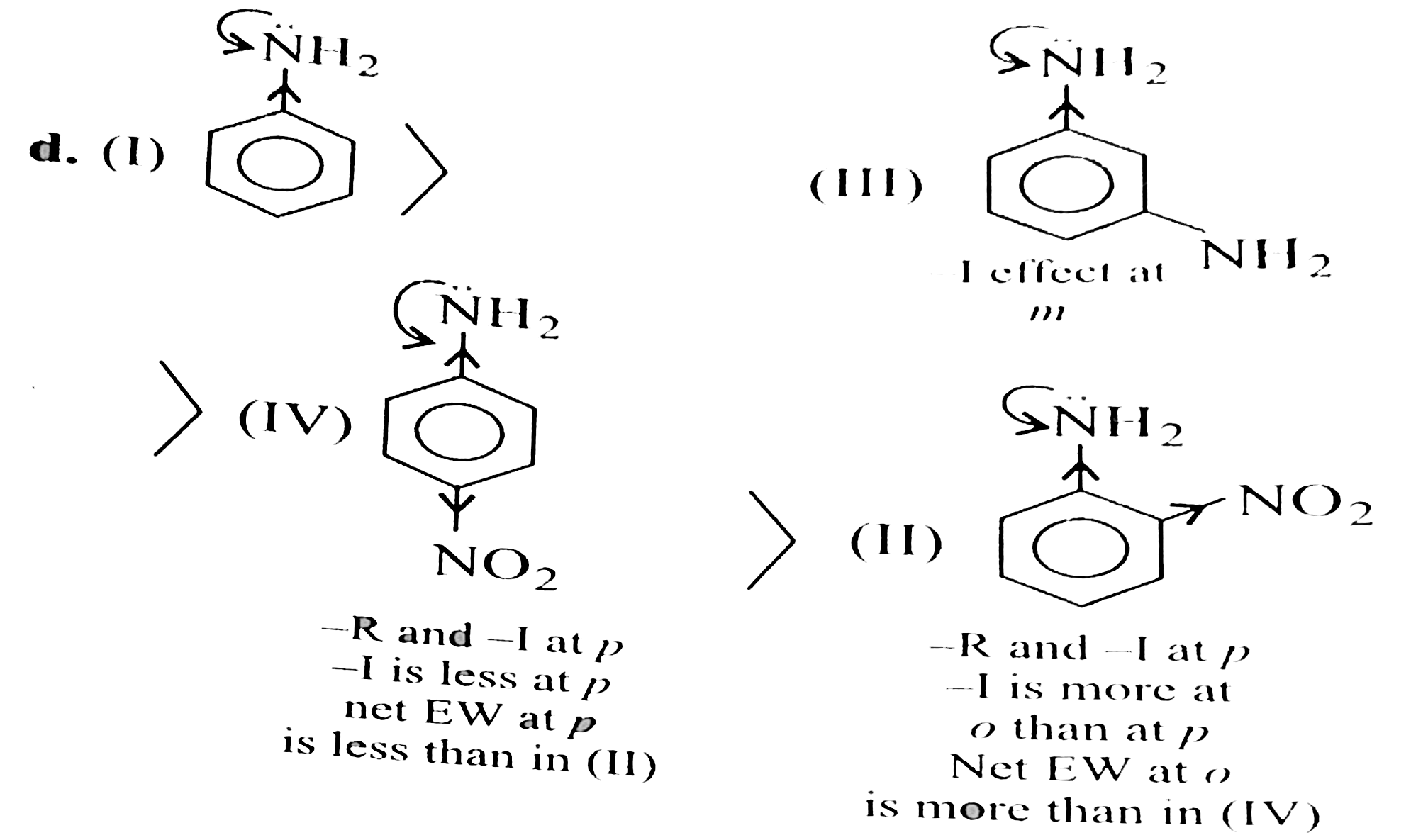
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?