Saved Bookmarks
| 1. |
Give the products of the following and the name of the reactions: |
Answer» Solution :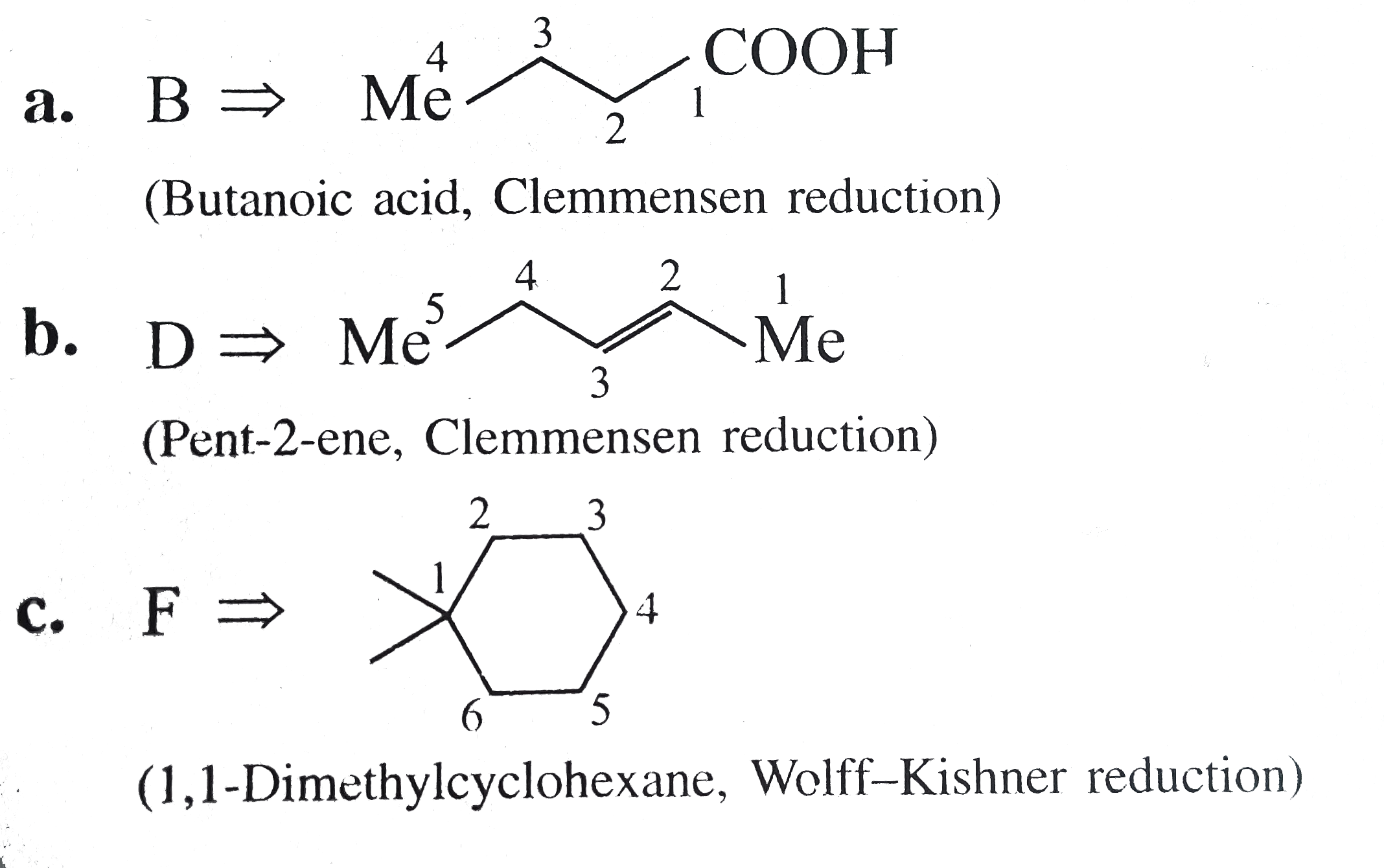 d. Clemmensen reduction is acid sensitive, so it would reduce `(C=O)` to `(-CH_(2))` GROUP and simultaneously dehydration of alcohol will OCCUR. 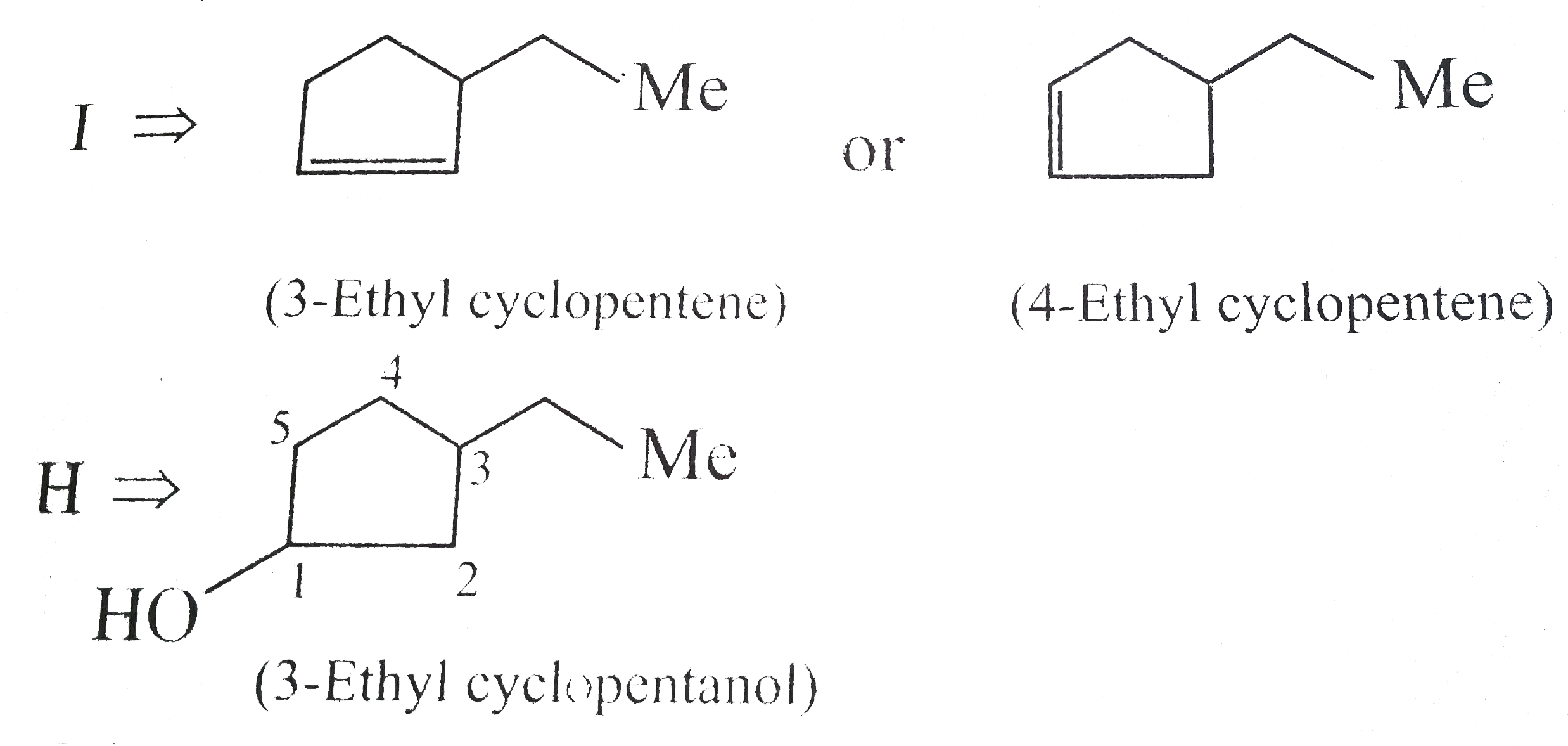 e. Wolff-Kishner reduction is base sensitive, so it would reduce `(C=O)` to `(-CH_(2)-)` group and simultaneously dehydrohalogenation of `RX` will occur. 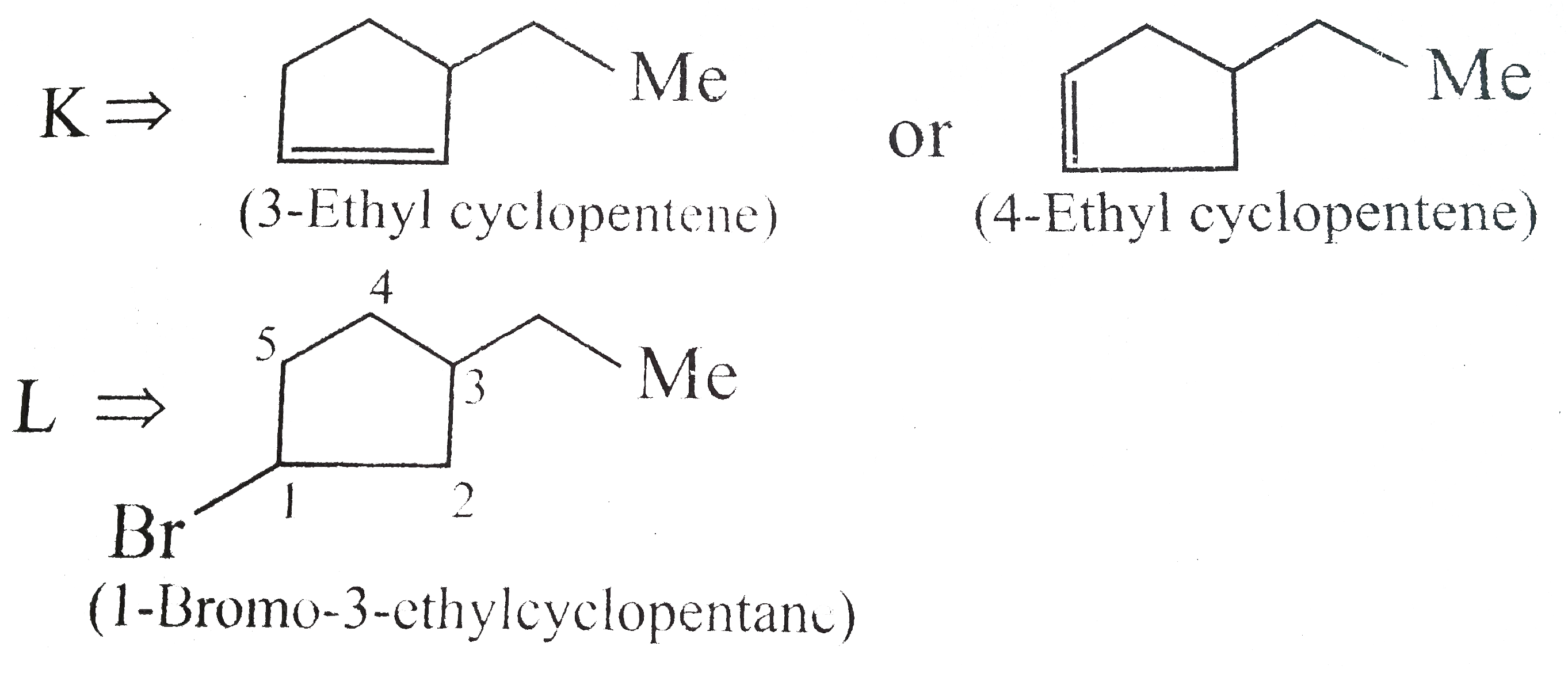 f. Both `LAH` and `NaBH_(4)` reduce `(-CH=O)` group tp `(-CH_(2)OH)` group. But `lAH` reduces `1^(@)` and `2^(@) RX` to `RH`, but `NaBH_(4)` does not reduce `1^(@) RX`, whereas both `LAH` and `NaBH_(4)` do not reduce `ArX`. 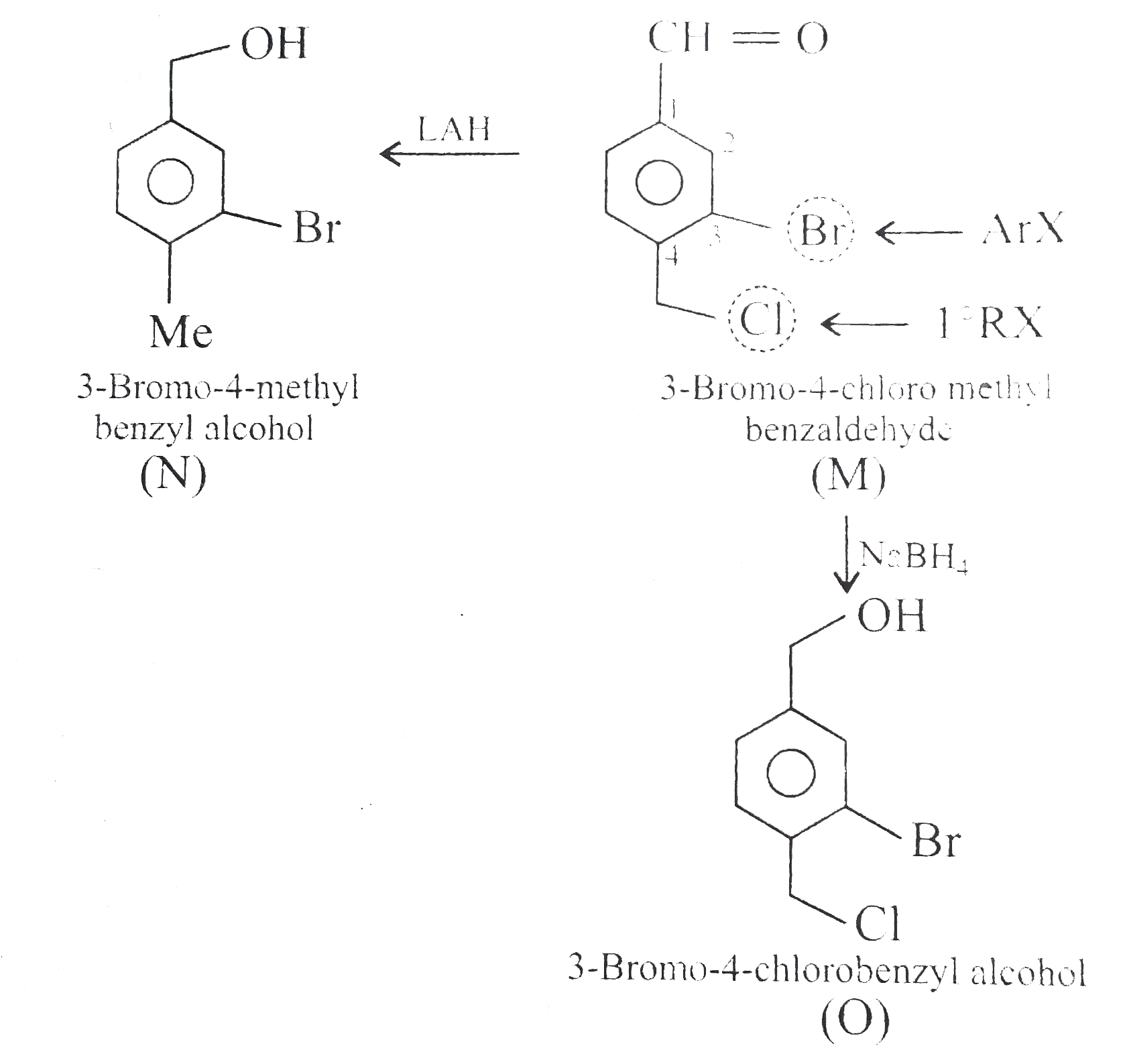 g. `LAH` reduces `R-NO_(2)` (aliphatic nitro group) to `R-NH_(2)`, and `Ar-NO_(2)`(aromatic nitro group) to `Ar-N=N-Ar` (azo group), whereas `NaBH_(4)` does not reduce either of them. 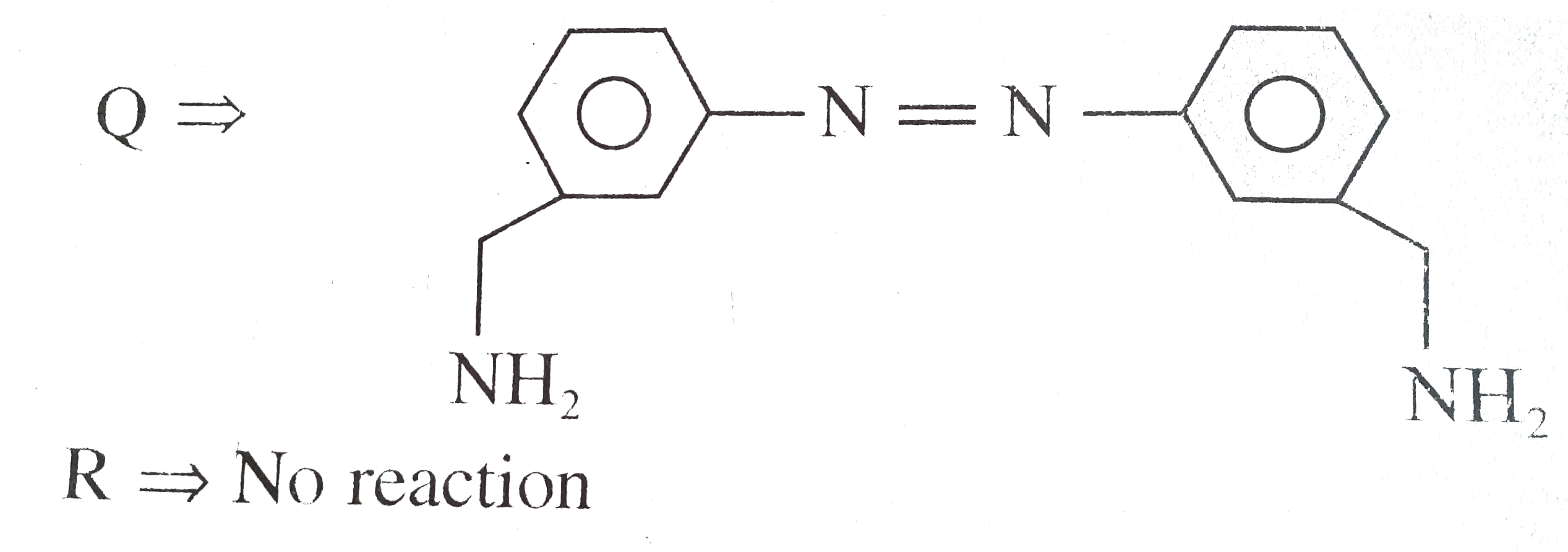 h. Ranet `Ni`, under high temperature and pressure, reduces all the double bonds of the benzene ring. 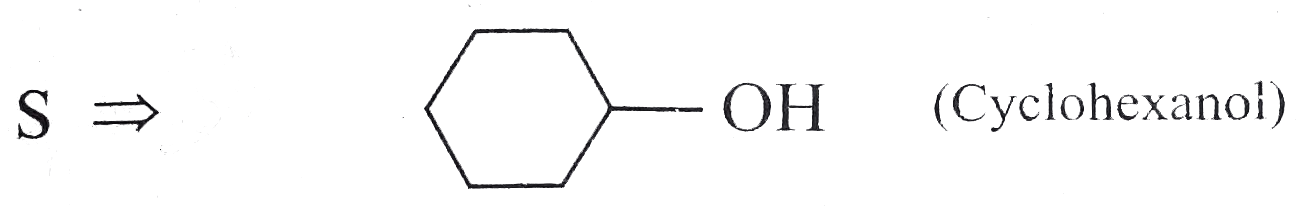 i. Under the given conditions, all the double bonds of the benzene are REDUCED. 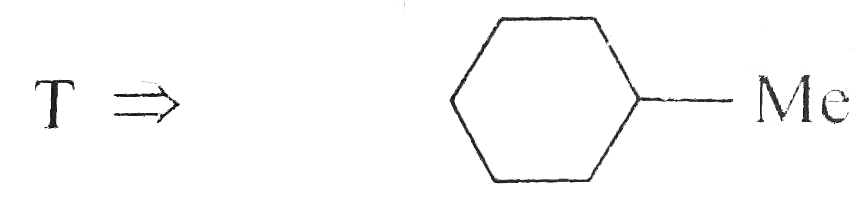 j. It is a Birch reduction and reduces one double bond of the benzene ring to give a compound containing containing isolated diene. 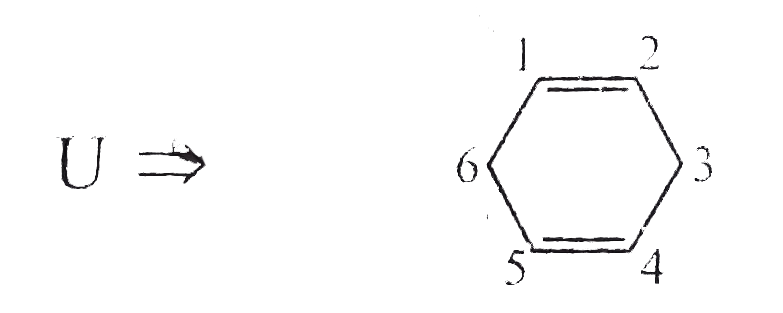 k. Lindlar's catalyst converts `(C=C)` to `(C=C)` bond to give cis-alkene `(syn-addition of H_(2))`, whereas Birch reduction `(Na+liq.NH_(3)+EtOH)` converts `(C-=C)` to `(C=C)` bond to give trans-alkene (anti-addition of `H_(2))` 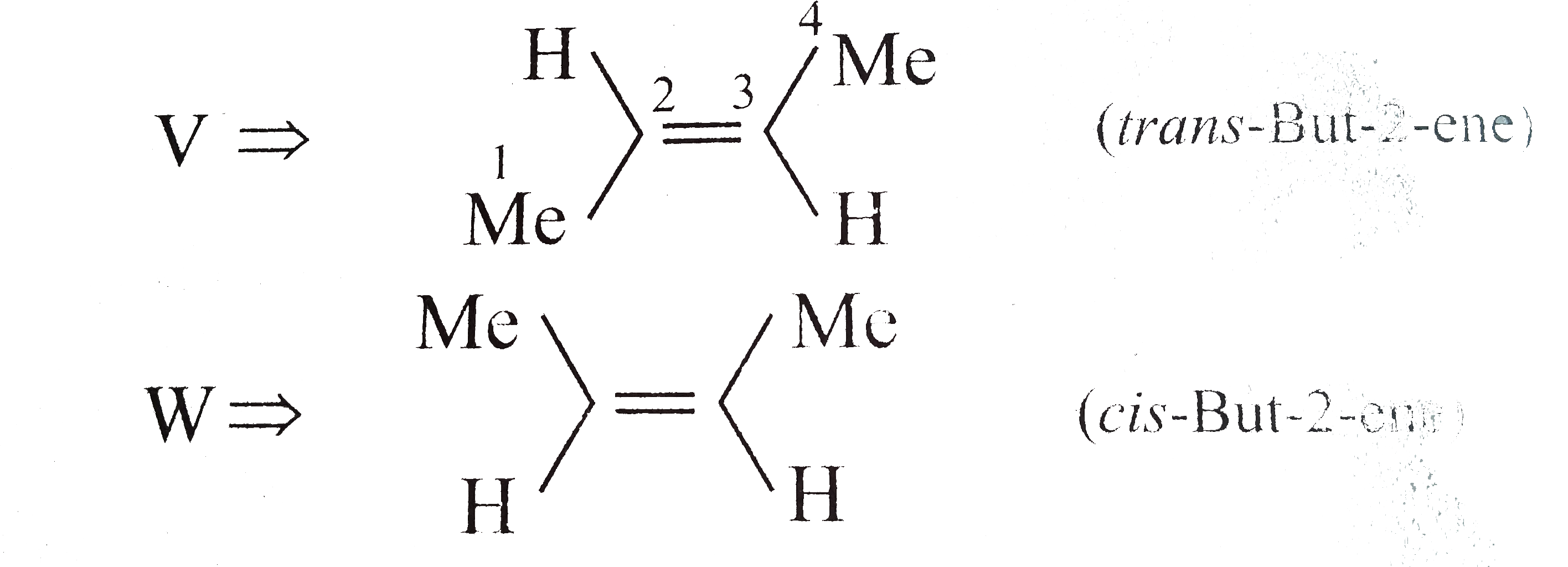
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?