Saved Bookmarks
| 1. |
How are 1-nitropropane, 2-nitropropane and 2-methyl-2- nitropropane are distinguished from each other using nitrous acid ? |
|
Answer» Solution :Primary, SECONDARY and tertiary nitroalkanes are distinguished on the BASIS of their REACTION with freshly prepared nitrous `(HNO_(2))` -a mixture of sodium nitrite and hydrochloric acid. `NaNO_(2)+HCl to NaNO_(2)+HNO_(2)` Primary nitroalkanes react with nitrous acid to from blue coloured nitroso - nitroalkanes (acid from) which dissolve in NaOH to given RED solutions.  Secondary nitroalkanes reach with nitrous acid to from blue coloured nitroso - nitroalkanes. As nitroso-nitroalkane do not have `alpha`- HYDROGEN atom hence are insoluble in NaOH. 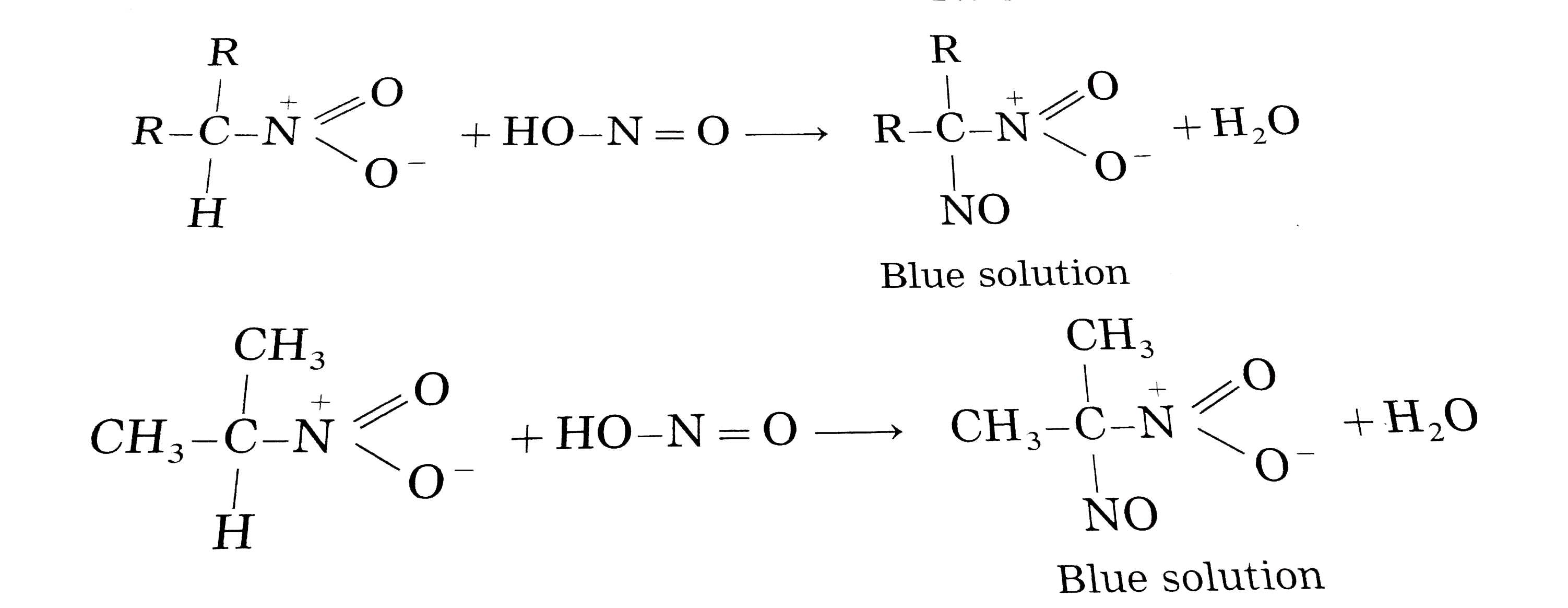 Tertiary nitroalkanes (2-methy1-2-nitropropane) do not react with nitrous acid since they do not contrain `alpha`-hydrogen atom. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?