Saved Bookmarks
| 1. |
How are polymers classified on the basis of intermolecular forces of attraction ? |
|
Answer» Solution :The classification of polymers on the basis of inter-MOLECULAR forces of atraction such as van der Waal.s forces and hydrogen bonding etc. can be done in the following way : (i) Elastomers . Such of polymers have weakest inter-molecular forces between the polymer chains. As a result of this stretching of chains can take place . However , there are a few cross-links also between the chains due to which the chains regain their original position when the stretching force is withdrawn. (ii) Fibres . Such of polymers have the strongest inter-molecular forces such as hydrogen bonds and dipole-dipole interactions. As a result of this, fibres have high tensile strength and ELASTICITY. 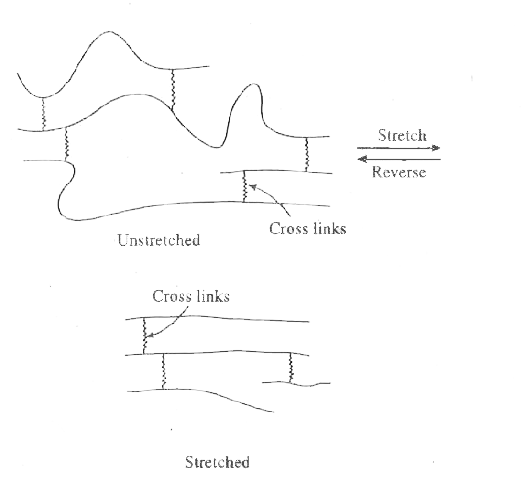 The molecules of fibres are long, thin and thread-like and can be woven into fabrics. Nylon-66 and terylene are EXAMPLES the magnitude of inter-molecular forces is intermediate between those in elastomers and fibres. These are no cross links between the polymer chains. As a result , thermoplastics are not very rigit and can be moulded into different shapes. Polythene , polypropylene and polystyrene are some examples of thermoplastics. (IV) Thermosetting polymers . Such polymers have relatively low molecular masses which get highly cross-linked on heating . This gives rise to a hard, infusible NAD insoluble three dimensional network soild. Bakelite and melamine are examples of thermosetting polymers. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?