Saved Bookmarks
| 1. |
How are the following conversions carried out ? 2-methylbutan-1ol-2methylbutanoic acid Pheylethene into Benzoic acid (3) Benzoic acid into metanitrobenzoic acid What is the action of Benzene sulphonyl chloride on primary, secondary and tertiary amines? Write two uses of formaldehyde. |
|
Answer» Solution :(1) 2-methylbutan-1-ol into 2-methylbutanoic acid `CH_(3) CH_(2) CH_(2) (CH_(3)) CH_(2) OH overset([O])(to) CH_(3) CH_(2) CH_(2) (CH_(3)) COOH` (2) PHENYL ethene into Benzoic acid  (3) Benzoic acid into metanitrobenzoic acid.  Benzene sulphonyl chloride is Hinsberg's REAGENT. It reacts with all the amines differently to form sulphonamide which can be used to distinguish between primary, SECONDARY and tertiary amines. Primary aminues react with benzenesulhonyl chloride formaing N-alkyl benzene sulphonamide. It is simply acid base type of reactions. Amine has lone pair of electrons on N, so it acts as base and attacks the electrophilic centre of the reagent that is S atom. Attack of `NH_(2)` helps in removal of `CI^(-)` and the product is sulphonamide. The primary amine sulphonamide is soluble in alkali. 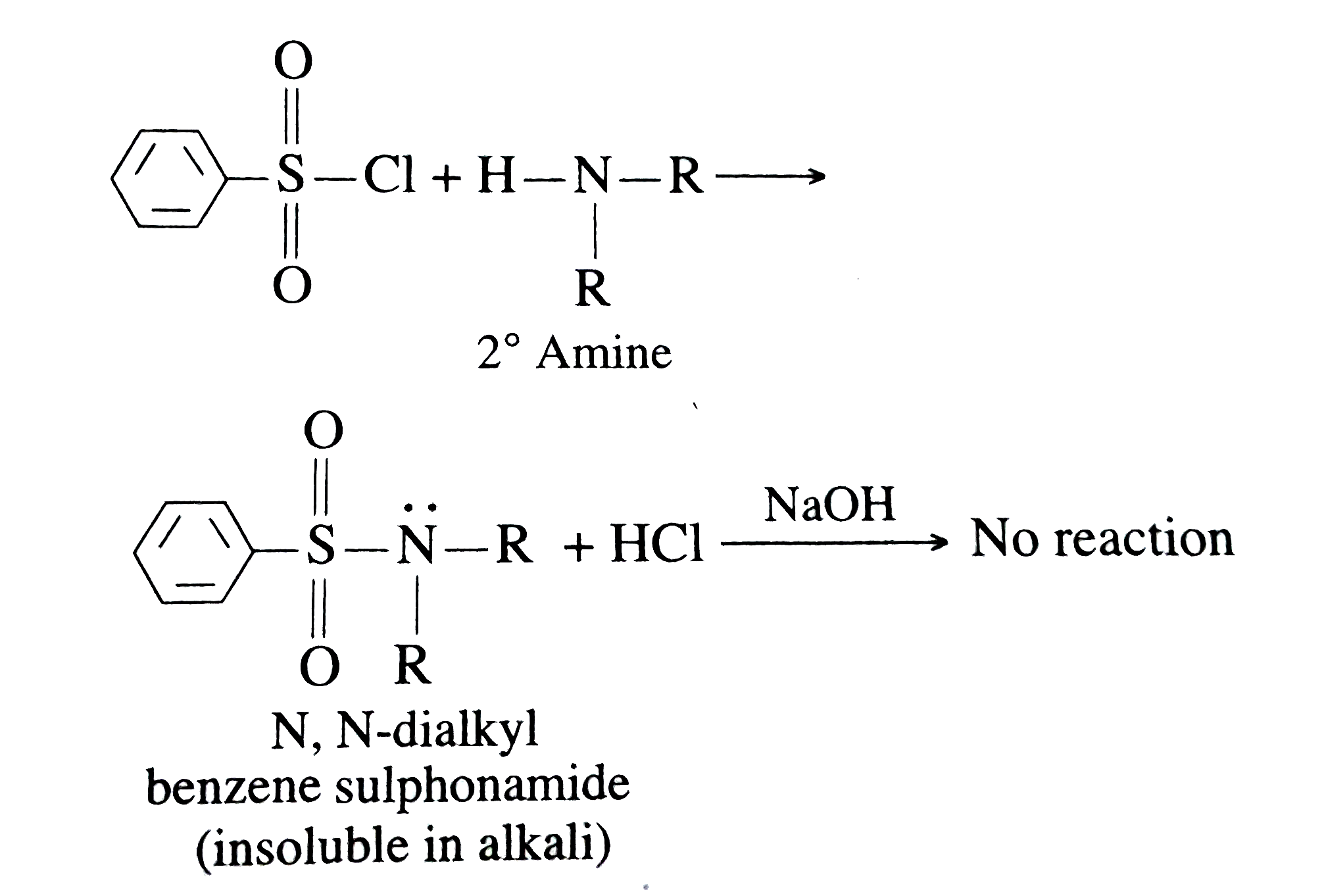 Secondary amines reacts with Benzenesulphonyl chloride FORMING N, N-dialkyl benzene sulphoanmide which is insoluble in alkali.  Tertiary amines do not react with benzenesulphonyl chloride Formaldehyde is regularly used in the chemical medical industries and it has preservative and sterilizing properties. It is also used to treat various types of building materials and in the creation of foam insulation and some types of fabrics. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?