Saved Bookmarks
| 1. |
How do you explain the geometry of the molecules on the basis of Valence bond Theory ? |
|
Answer» Solution :The valence bond theory explains the SHAPE, the formation and directional properties of bonds in poly atomic molecules like `BeCl_(2), BCl_(3), CH_(4), NH_(3), H_(2)O`, etc. in terms of overlap and hybridisation of atomic ORBITALS. E.g., in `BeCl_(2)`, Be is the central atom. Its atomic number is 4. Therefore its ground state electron configuration is `1s^(2)2s^(2)`. In order to explain the formation of `BeCl_(2)`, SP hybridisation is to be assumed for Be in its excited state. The excited state configuration of Be is `1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(0)2p_(z)^(0)`. As a result of which two sp HYBRID orbitals will form on it. Now the two sp hybrid orbirtals overlap Head-Head with `3p_(z)` orbitals of two chlorine atoms forming two sigma bonds. The molecule is linear and the bond angle is `180^(@)`.  In `BCl_(3)` the central atom is boron. Its atomic number is 5. Therefore its ground state electron configuration is `1s^(2)2s^(2)2p_(x)^(1)2p_(y)^(0)2p_(z)^(0)`. Form this configuration it is evident that it exhibits mono-valency. The first excited state configuration of B is `1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(0)2p_(z)^(0)`. Since, there are two half-filled orbitals, the valency of Boron is 3. In order to explain the formation of `BCl_(3)` molecule `sp^(2)` hybridisation is to be assumed to boron atom in its excited state I. As a result of which three `sp^(2)` hybrid orbitals will form on it. Now, the three `sp^(2)` hybrid orbitals of 'B' atom overlap Head - Head with `3p_(z)` orbitals of three Cl atoms forming three sigma bonds. 'The shape of the molecule is plane triangular is plane triangluar and the bond angle is `120^(@)`.  `CH_(4)` (Methane) : In `CH_(4)` the central atom is carbon. Its atomic number is 6. Therefore the electron configuration is `1s^(2)2s^(2)2p^(2)(2p_(x)^(1),2p_(y)^(1),2p_(z)^(@))`. In order to explain the formation of four bonds excited state configuration `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(1)2p_(z)^(1))` is to be taken. From this configuration, we can say that carbon can form four bonds. `[Ex. CH_(4)]` 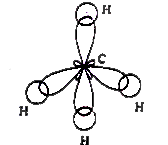 In the formation of methane molecule the central carbon atom undergoes `sp^(3)` hybridisation in its excited state `(1s^(2)2s^(1)2p_(x)^(1)2p_(y)^(1)2p_(z)^(1))`. As a result of which four `sp^(3)` hybrid orbitals form on it. Each of them contains an unpaired electron. Now, these four hybrid orbitals overlap Head-Head with 1s orbitals of four hydrogen atoms forming `CH_(4)` molecule. The strucrture of the molecule is tetrahedral. The bond angle is `109^(@)28'`. Since there are no lone pairs, there is no DISTORTION in the structure of the molecule. `NH_(3)` (Ammonia) in `NH_(3)` molecule, one `sp^(3)` orbital contains a lone pair of ELECTRONS. There will be repulsions between the lone pair and bond pair electrons. These are stronger than bp - bp repulsions. `(l.p-b.p gt b.p-b.p)`. So the bond pairs are pushed together. Hence the bond angle decreases from tetrahedral angle. The molecule assumes a pyramidal shape. 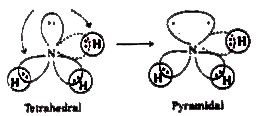
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?