Saved Bookmarks
| 1. |
Pratap is a student of class IX in a city school. He has recently studied the writting of formulae of ionic compounds in the class. Pratap has made some placards for a science quiz showing the symbols of some elements and some ions but forgot to write the electric charges on the ions. Moreover, the placards made by Pratap got mixed up as shown below : Based on these placards, the chemistry teacher, Mr. Suri, asked Pratap to answer the following question : (a) Choose the symbol of the element from among the placards which can form divalent and trivalentcations. Name this element. Also write the symbols of these cations along with their charges (b) Choose the symbol of a divalent anion from among the given placards. what is the name of this anion. Also write the symbol of this anion alongwith its charge (c) Work out the formula of the ionic compound formed between the divalent cation and divalent anion described above. Also name the compound formed (d) Work out the formula of the ionic compound formed between the trivalent cation and divalent anion described above. Also name the compound formed (e) What values are displayed by Pratap in this episode ? |
|
Answer» Solution :(a) The positively charged ions are called cation. The symbol of element which can form divalent cations. (valency 2+ ions) and trivalent cations (valency 3+ ions) is Fe. The name of the this element is iron. The symbols of two cations formed by iron element are `Fe^(2+)` [ferrous ion or iron (II) ion] and `Fe^(3+)` ion [ferric ion or iron (III)ion] (b) The negatively charged ions are called anions. The symbol of the divalent anion (valency 2-ion) is `SO_(4)`. The name of this anion is sulphate ion. The symbol of sulphate ion ALONG with its charge is `SO_(4)^(2-)` (c) The divalent cation (or valency 2+ ion) is `Fe^(2+)` and the divalent anion (valency 2- ion) is `SO_(4)^(2-)`. The formula of compound formed from these ions can be worked out as follows : 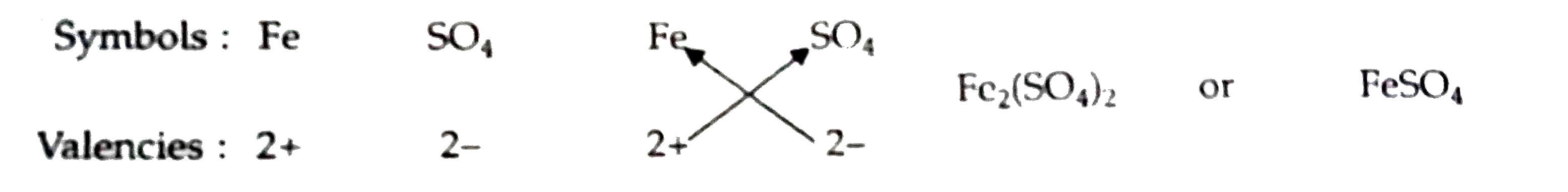 THUS, the formula of compound formed is `FeSO_(4)`. It is ferrous sulphate or iron (II) sulphate (d) The trivalent cation (or valency 3+ ion) is `Fe^(+)` and the divalent anion (or valency 2- ion) is `SO_(4)^(2-)`. The formula of compound formed from these ions can be worked out as follows : 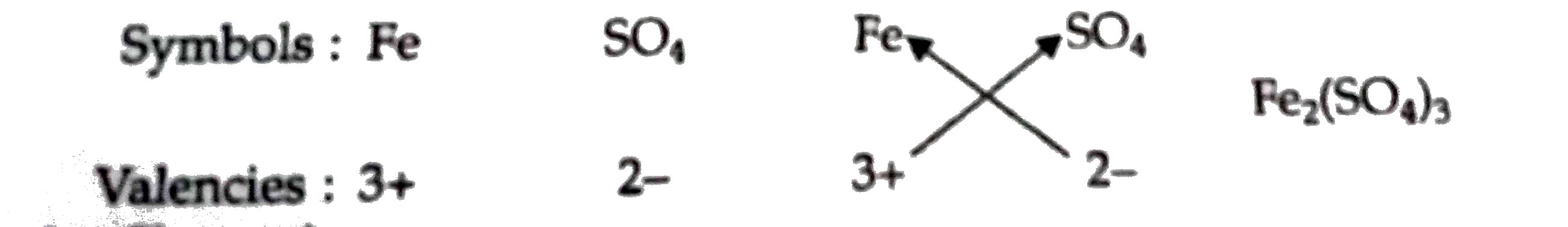 Thus, the formula of compound formed is `Fe_(2)(SO_(4))_(3)`. It is ferric sulphate or iron (III) sulphate (e) The VALUES displayed by Pratap in this episode are (i) Knowledge of cations (positive ions) and anions (negative ions), and their valencies, and (ii) ABILITY to use this knowledge in solving PROBLEMS. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Two ice blocks of 10 g each are placed in 2 L distilled water at 273 K. One of theice blocks is made up of distilled water. What will you observe if the ambient temperature is also 273 K ? Give reasons to support your observation.
- Which separation techniques will you apply for the separation of the following ? (a) Sodium chloride from its solution in water. (b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride. ( c) Small pieces of metal in the engine oil of a car. (d) Different pigments from an extract of flower petals. (e) Butter from curd. (f) Oil from water. (g) Tea leaves from tea. (h) Iron pins from sand. (i) Wheat grains from husk. (j) Fine mud particles suspended in water.
- The teacher instructed three students 'A', 'B' and 'C'respectively to prepare a 50% (mass by volume) solution of sodium hydroxide (NaOH). 'A' dissolved 50g of NaoH in 100 mL of water. 'B' dissolved 50g of NaOH in 100g of water while 'C' dissolved 50g of NaOH in water to make 100 mL of solution. Which one of them has made the desired solution and why?
- What are impure substance? Give example.
- Why are cotton clothes prefferred in summer ?
- What is the effect of heat on the rate of diffusion?
- What is the mass of 1 mole of nitrogen atoms ?
- What is superphosphate of lime ?
- CHARACTERISTICS OF PARTICLES OF MATTER
- The separation of denser particles from lighter particles done by rotation at high speed is calls _______