Saved Bookmarks
| 1. |
Predict the conditions under which (a)Aluminium might be expected to reduce magnesia (b)Magnesium could reduce alumina. (ii)Carbon monoxide is more effective reducing agent than carbon below 983 K but, above this temprature,the reverse is true -Explain. (iii)it is possible to reduce Fe_(2)O_(3) by coke at a temprature around 1200 K. |
|
Answer» Solution :(i)The CONDITIONS under which: (a)Ellingham diagram is used to PREDICT thermodynamic feasiblity of reduction of OXIDES of one metal by another metal.Any metal can reduce the oxides of other metals that are located above it in Ellingham diagram.In the Ellingham diagram,for the formation of magnesia (magnesium oxide) occupy lower than aluminium oxide.therefore aluminium cannot be used to reduce the oxides of magnesium (magnesia).Above 1623k,Al can reduce MgO to Mg,so that `Delta_(r)G^(@)` becomes negative an the process becomes thermodynamically feasible. `3MgO+2Aloverset(1623K)(to)Al_(2O_(3))+3Mg` (b) 1.`(4)/(3)Al+O_(2)to(2)/(3)Al_(2)O_(3)`2.`2Mg+O_(2) to 2MgO` At the point of intersection of the `Al_(2)O_(3)` and MgO curves in Ellingham diagram. `DeltaG^(@)` becomes zero for the reaction: `(2)/(3)al_(2)O_(3)2Mgto2MgO+(4)/(3)Al` Below that point magnesium can reduce alumina. (ii) From the Ellingham diagram,we find that at 983 K,the curves intersect. 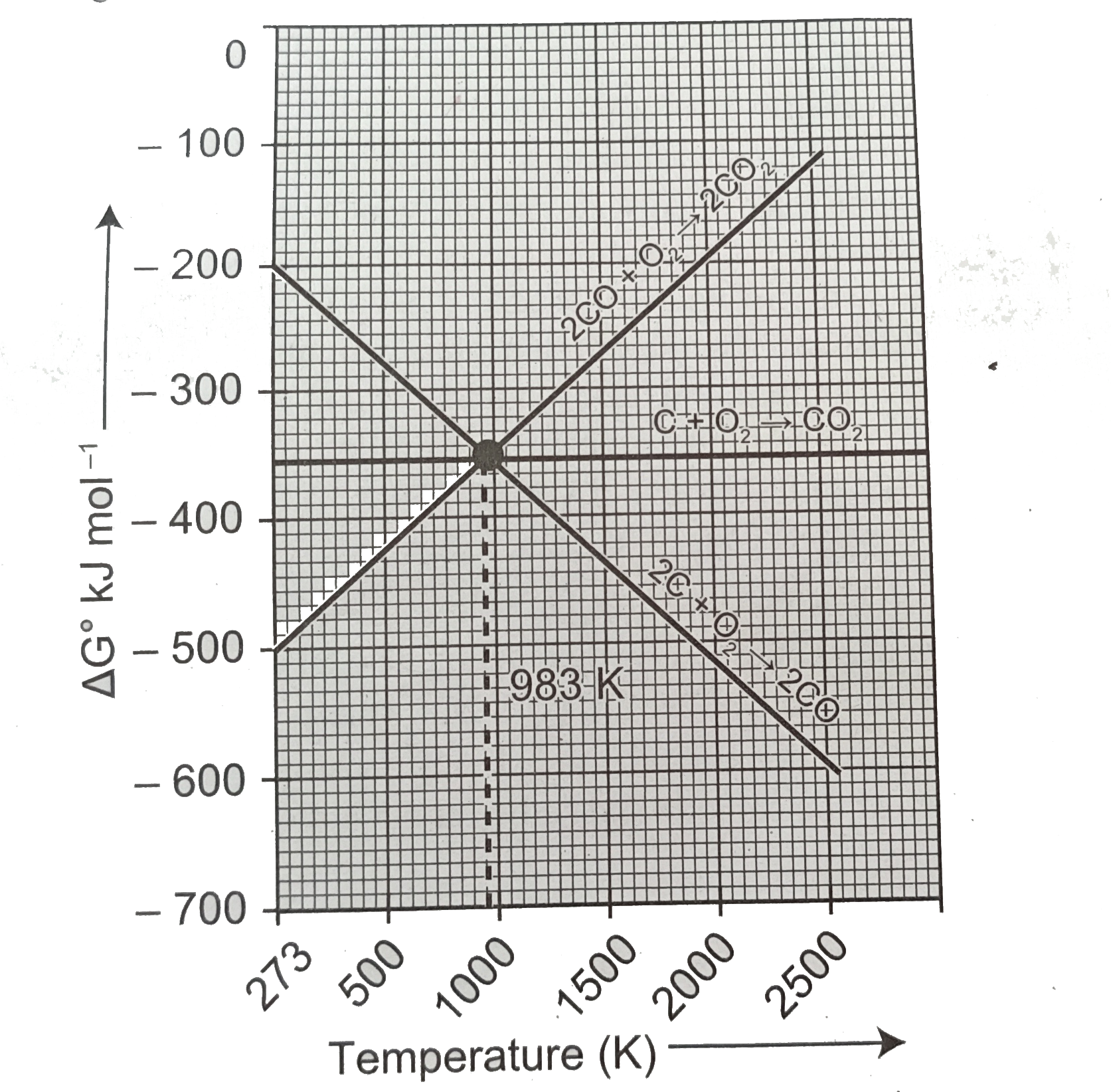 The value of `DeltaG^(@)` for change of C to `CO_(2)` is less than the value of `DeltaG^(@)` for change of CO to `CO_(2)`.therefore ,coke (C) is a better reducing agent than CO at 983K or above temprature.However below this temprature (e.g. at 673K), CO is more effective reducing agent than C. (iii)(a)Yes,it is possible to reduce `Fe_(2)O_(3)` by coke at a temprature arounf 1200K (b)In the Ellingham diagram ,carbon line cuts across the lines of many metal oxides and hence it can reduce all those metal oxides at sufficiently high temprature.Ellingham diagram for the formation of `Fe_(2)O_(3)` and CO intersects around 1000K.Below this temprature ,the carbon line lies above the iron line which indicates that `Fe_(2)O_(3)` is more stable than CO and hence at this temprature range the reduction is not line and hence we can use coke as a agent arounf 1200 K. (C)Around 1200 K,coke is better reducing agent because above 1000 K,Gibb,s free energy for the formation of `Fe_(2)O_(3)` is more than the formation of `CO_(2)` from C. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?