Saved Bookmarks
| 1. |
Predict the valencies of the elements with atomic numbers 7 , 15 , 16 , 18 , 19 and justifyAlso give the formulae of hydrides formed by the above elements if any. |
Answer» SOLUTION :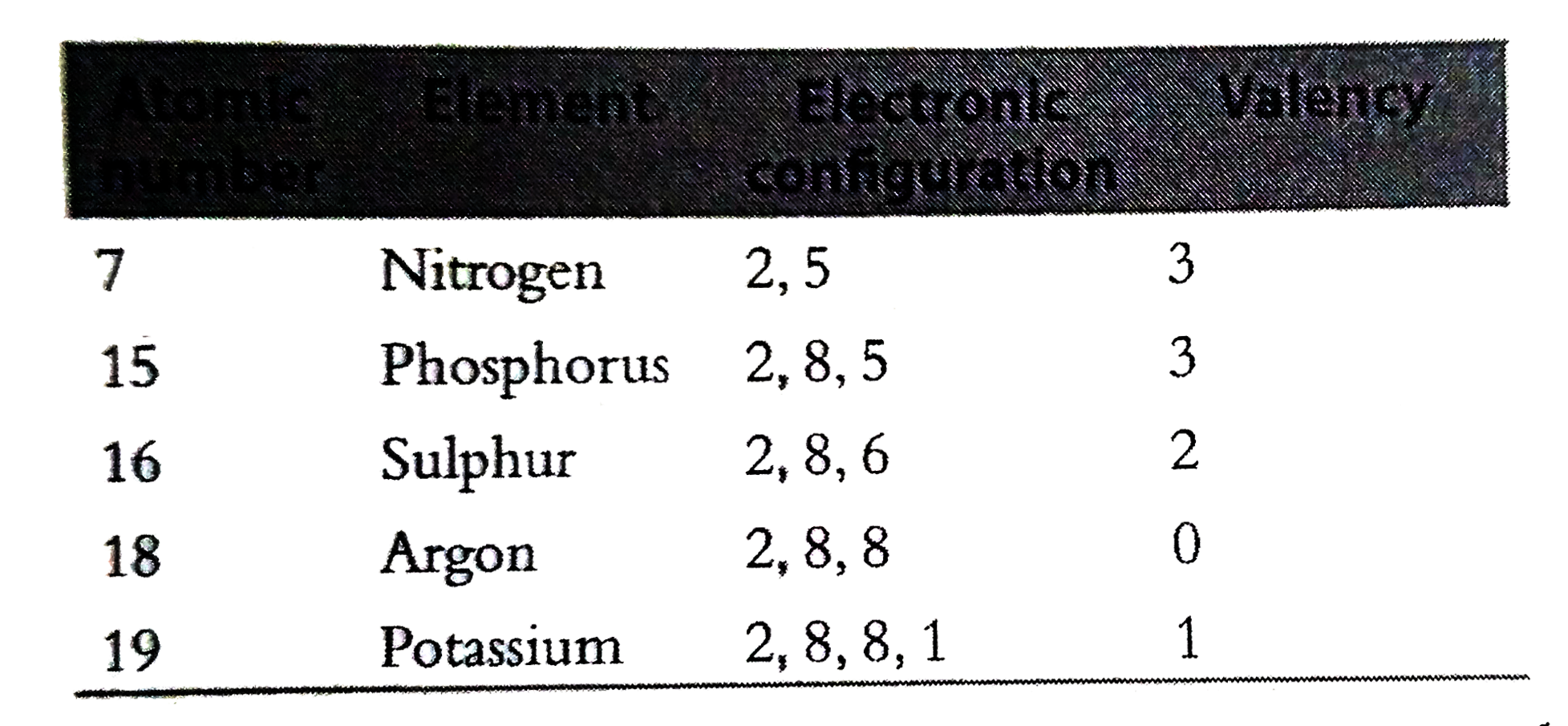 Since N andP need three more electrons to ATTAIN octet , their valencies are 3. Similarly , the valencies of other elements with atomic NUMBER 16 , 18 , 19 are 2 , 0 and 1 respectively. Except argon , all other elements forms hydrides. Phosphorus`- PH_(3)`, Potassium- KH Nitrogen`NH_(3)` SULPHUR`- H_(2)S` Argon does not from any COMPOUND |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following phenomenon is an effect acid rain ?
- Which of the following can be used to reduce suspended particulate matter in atmosphere in mine areas ?
- What are noble gases ? Mention their uses.
- What will be the formula of the sulphate and sulphite of a trivalent metal that isM^(+3) ?
- Which among the following is an element ?
- Xenon and krypton is used in electric bulbs to slow down the sublimation of tungsten.
- Whichof the following substances does not form a curdy precipitate when it is added to hard water ?
- Why are droplets of water observed on the walls of a glass tumbler containing ice ?
- What are homogeneous and heterogeneous mixtures ? Give one example for each.
- Which among the following is true regarding aqueous solution of sulphur trioxide and sodium oxide?