Saved Bookmarks
| 1. |
Prove that H_2SO_4 is a strong dibasic acid. (ii) Distinguish tetrahedral and octahedral voids. (b) In an octahedral crystal field , draw the figure to show splitting of d orbital's. |
|
Answer» Solution :(a) (i) Sulphuric acid forms two types of salts namely sulphates and bisulphates . `H_2SO_4+NaOHrarrunderset("sodium bisulphate")(NaHSO_4)+H_2O` `H_2SO_4+2NaOHrarrunderset("sodium sulphate")(Na_2SO_4)+2H_2O` `H_2SO_4+2NH_3rarrunderset("Ammonium sulphate")((NH_4)_2SO_4)` 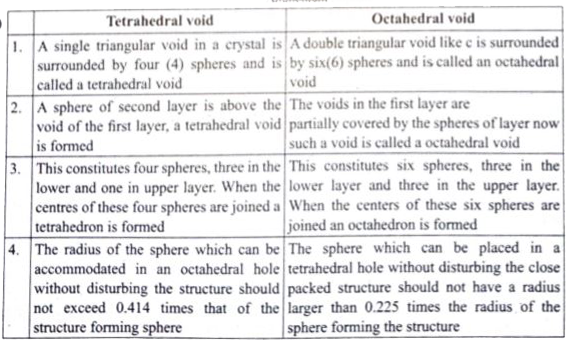 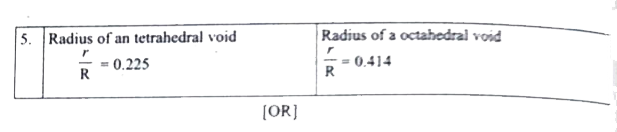 [OR] b. Step I : In an isolated gaseous state, all the five d orbital of the CENTRAL metal ion degenerate. Initially, the ligands from a spherical filed of negative charge around the metal, in this field the the energies of all the five orbitals willincrease due to the repulsion between the electrons of of the metal andthe ligand. Step 2: The ligands are approaching the metal atom in actual bond directions. To illustrate this letus consider and octahedral field, in which the central metal ionsis location at the origin and the six ligandsare COMING from the `+x,-x,+y,-y,+z and -z` direction as SHOWN below. . As shown in the figure,theorbitals lying along the AXES `dx^2-y^2 and dz^2` orbitals will experience strong repulsion and raise in energy to greater extent then the orbitals with lobes direction between the the axes `(d_(xy),d_(yz) and d_(zx))`Thus the degenerate d orbitals now split into two sets and the process is called crystal field splitting . 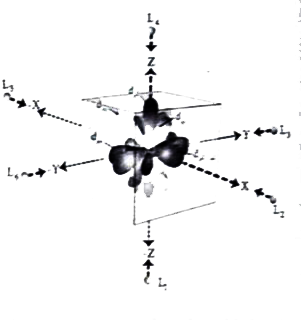 Step 3 : Up to this point the complex formation WOULD not be favoured. However , when the ligands approach further , there will be an attraction between the negatively charged electron and the positively charged metal ion, that results in a net decrease in energy.This decrease in energy is the driving force for the complex formation During crystal field splitting in octahedral field, in order to maintain the average energy of the orbitals (barycentre ) constant , the energy of the orbitals `d_(x^2-y^2)and d_(z^2)` (represented as `e_g` orbitals ) will increase by `3//5Delta_@`while that of the other three orbitals `d_(xy),d_(yz) and d_(zx)`( represented `t_(2g)`orbitals) decrease by `2//5Delta_@`Here, `Delta_@` represents the crystal field splitting energy in the octahedral field. 
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?