Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 27901. |
The metal that does not displace hydrogen from an acid is: |
| Answer» Answer :A | |
| 27902. |
The most stable form of sulphur at room temperature is |
|
Answer» monoclínic SULPHUR |
|
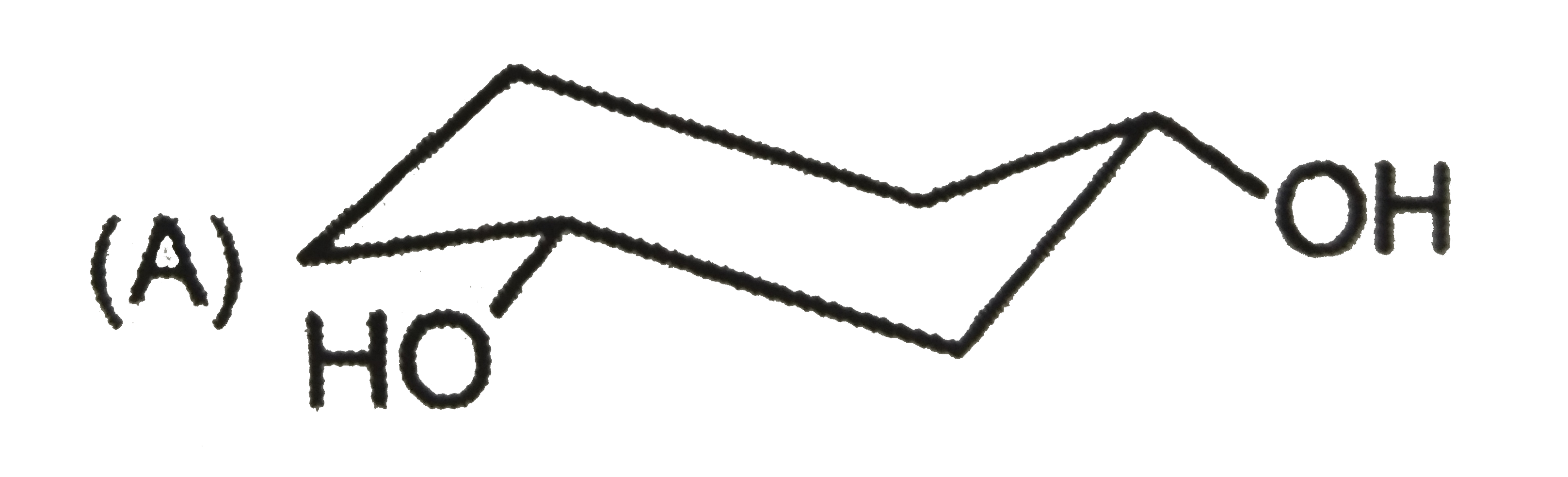
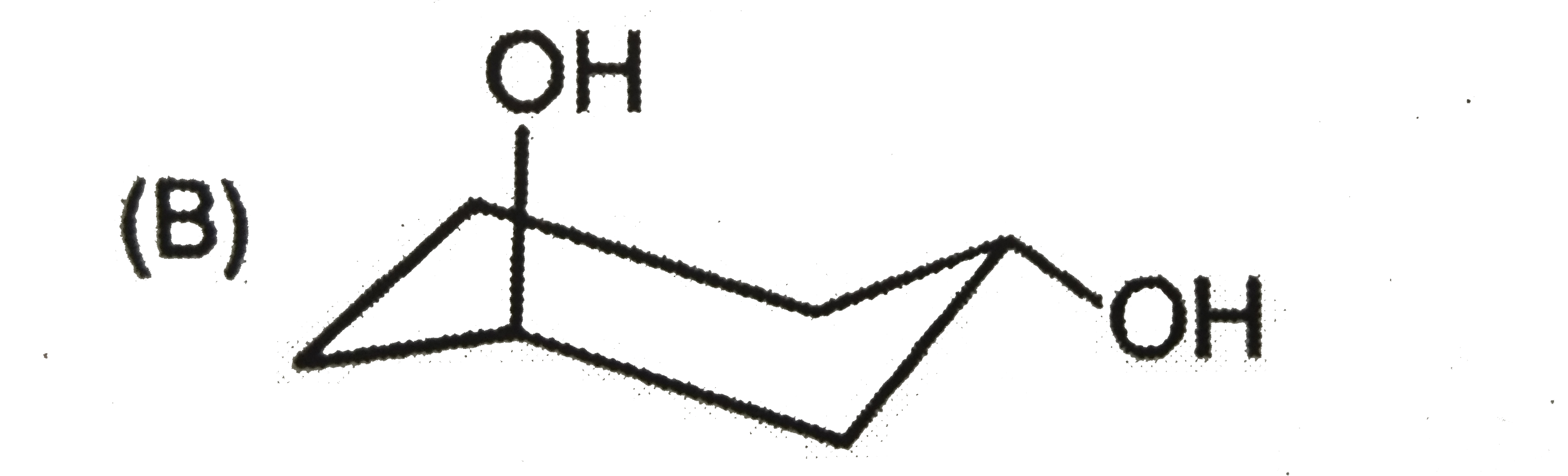
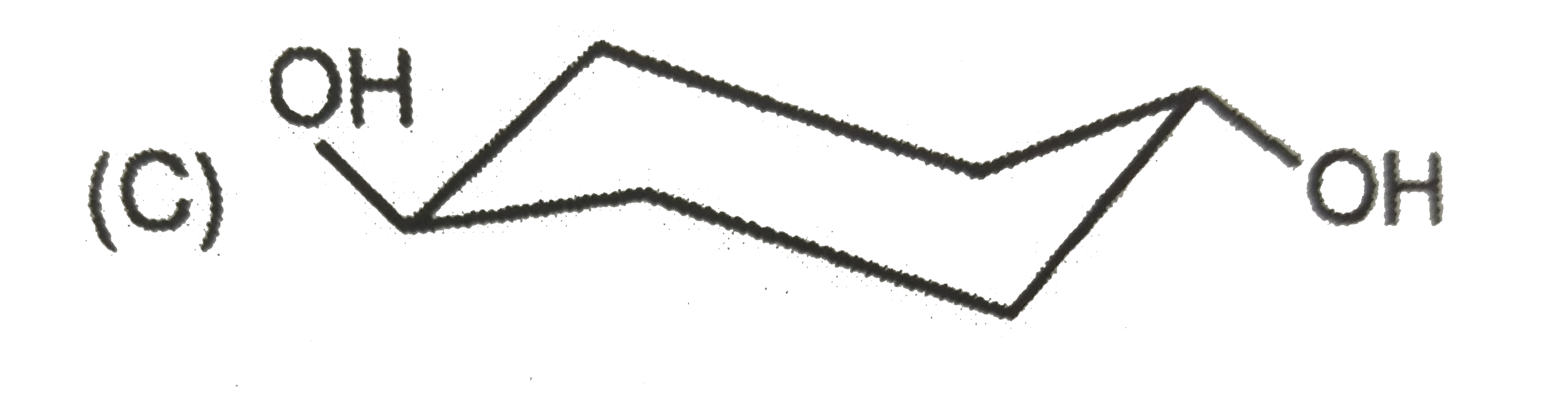
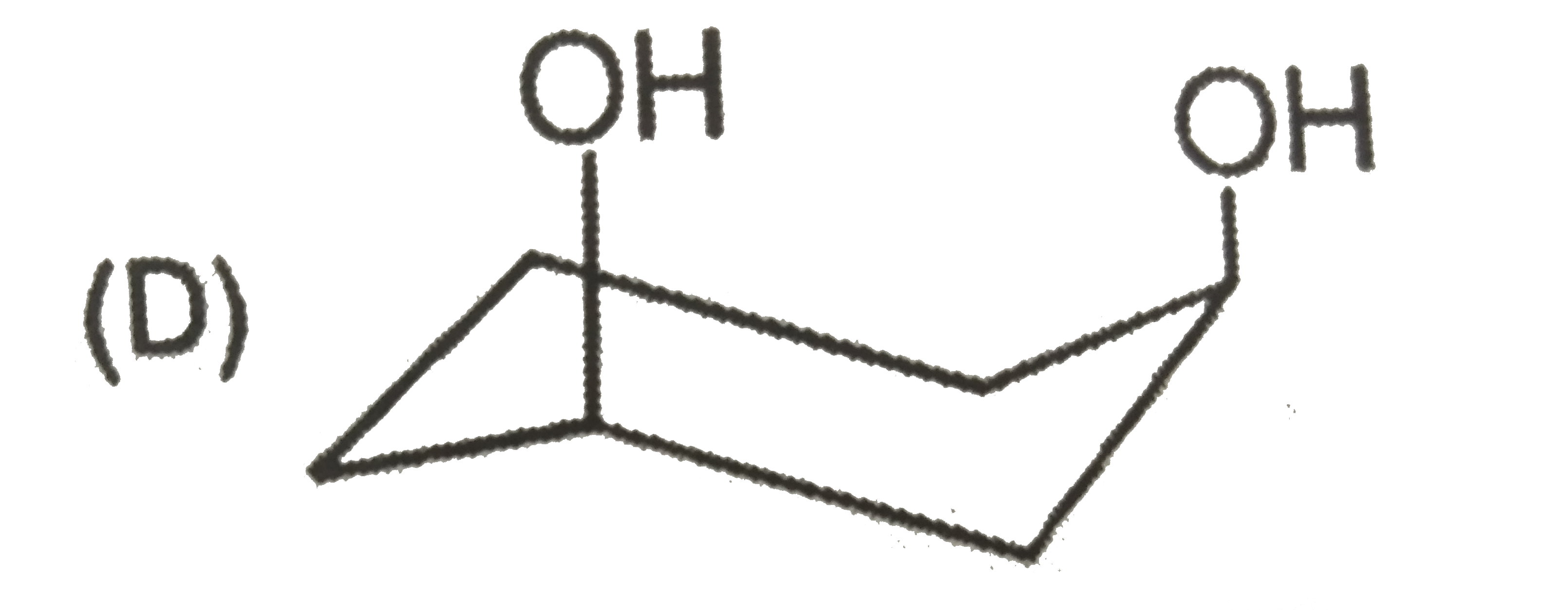
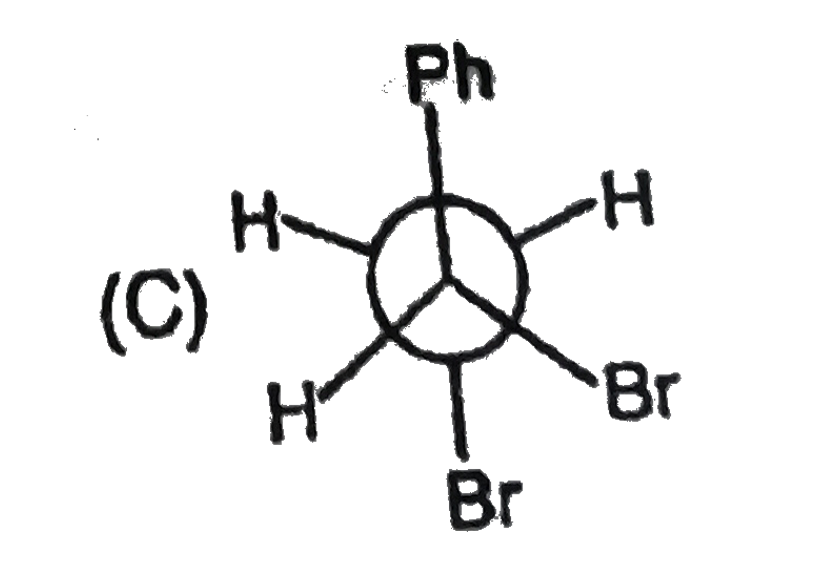
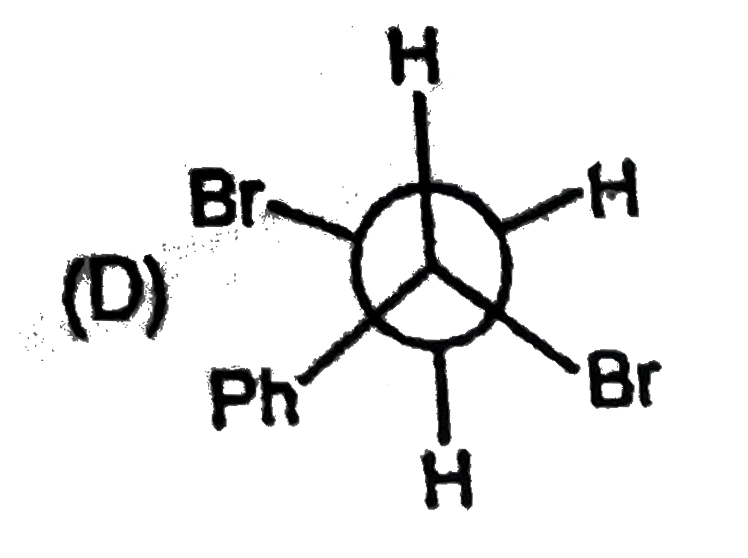
| 27903. |
The most stable form of cis cyclohexane -1,3-diol is represented as :1 |
|
Answer»
|
|
| 27904. |
The metal that does not displace hydrogen from an acid is : |
|
Answer» Hg |
|
| 27905. |
The most stable form of meso-tartaric acid is |
|
Answer» GAUCHE FORM 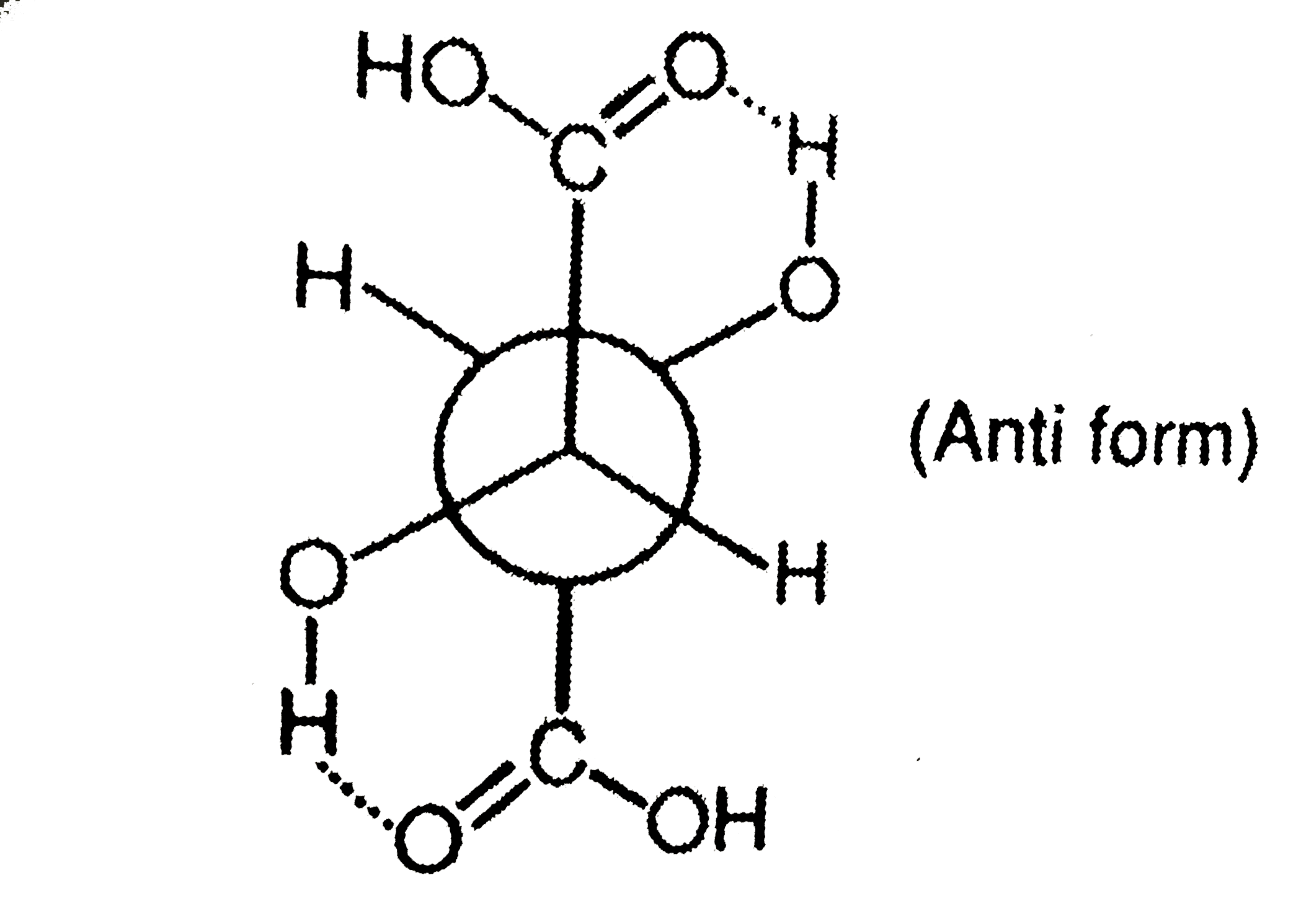
|
|
| 27906. |
The metal that cannot displace hydrogen from dilute hydrochloric acid is |
|
Answer» ALUMINIMUM |
|
| 27907. |
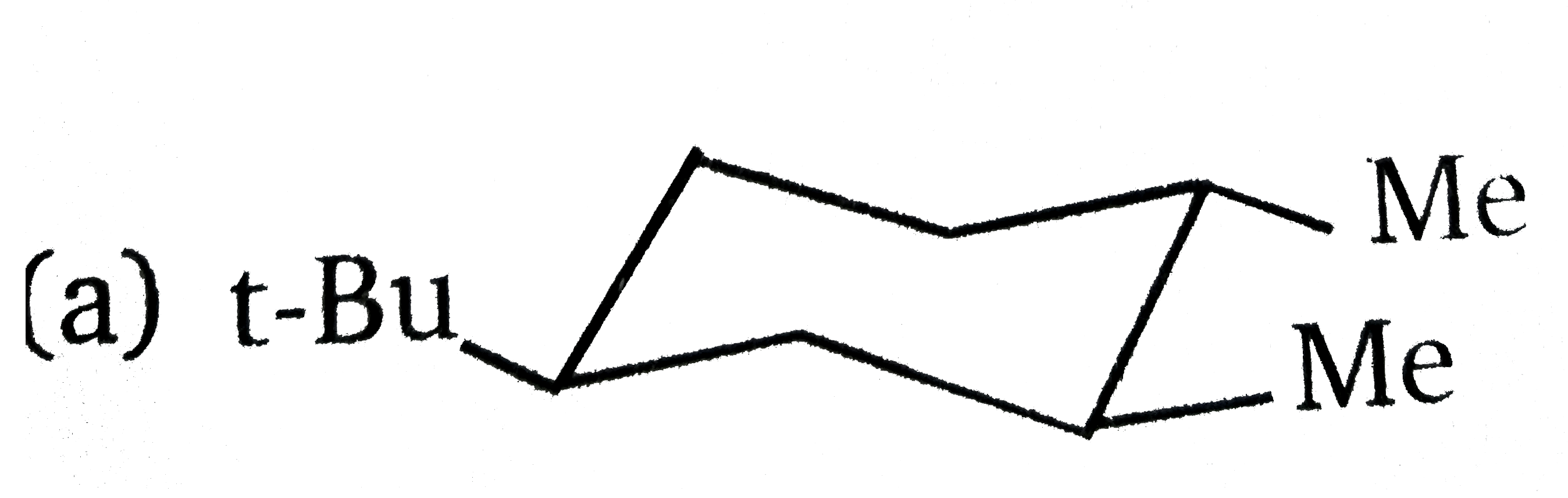
The most stable conformation of the following compound is : |
|
Answer»
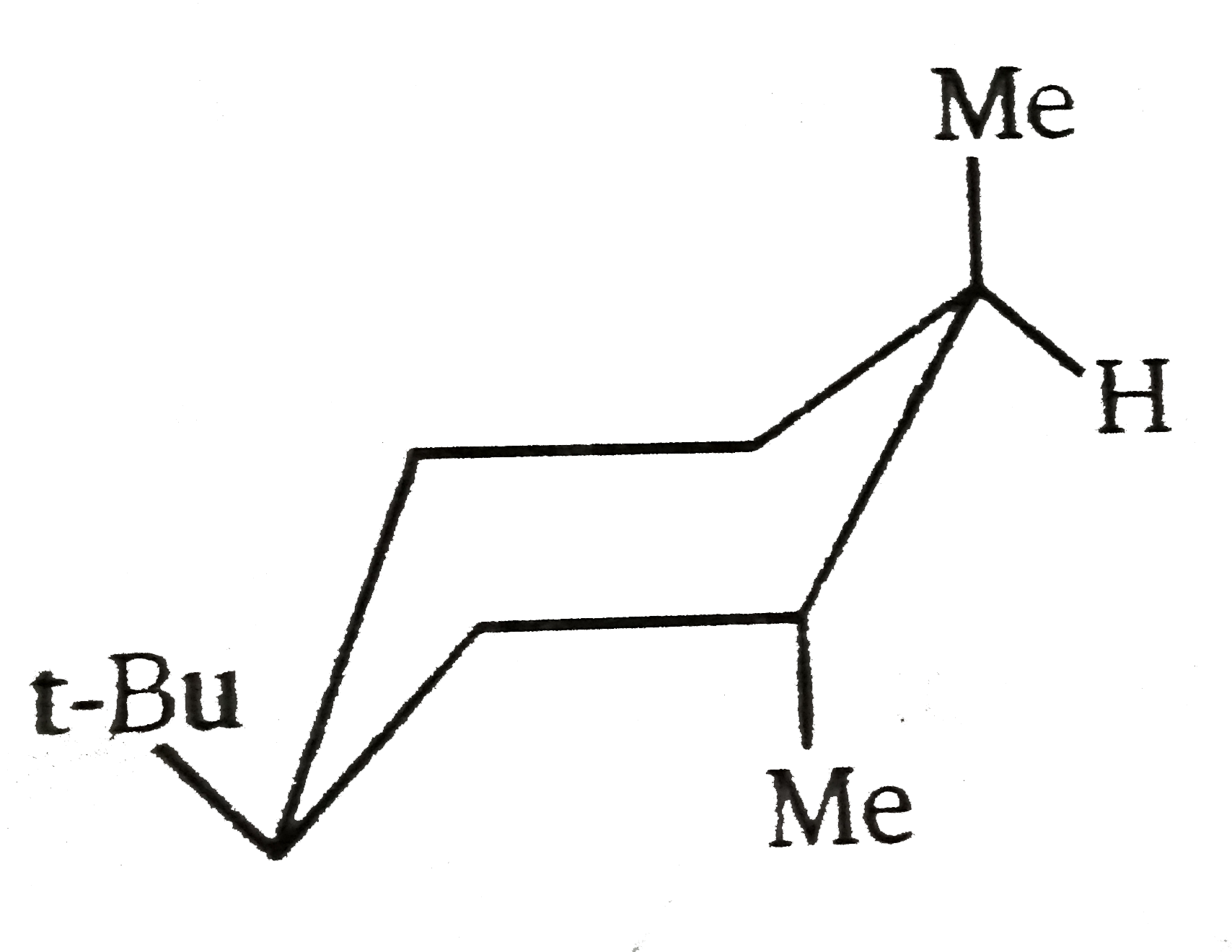
|
|
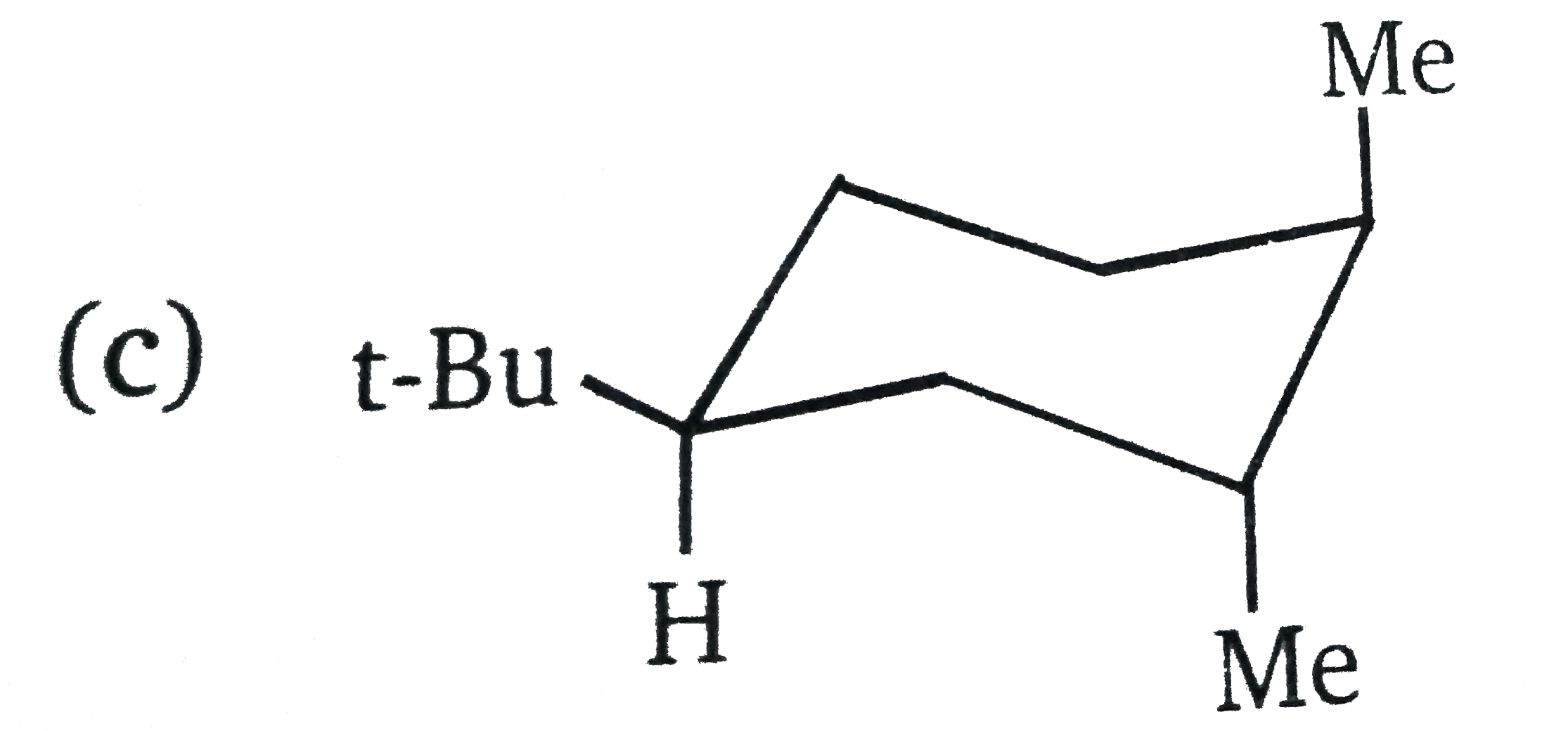
| 27908. |
The most stable conformation of the product of following reaction |
|
Answer»
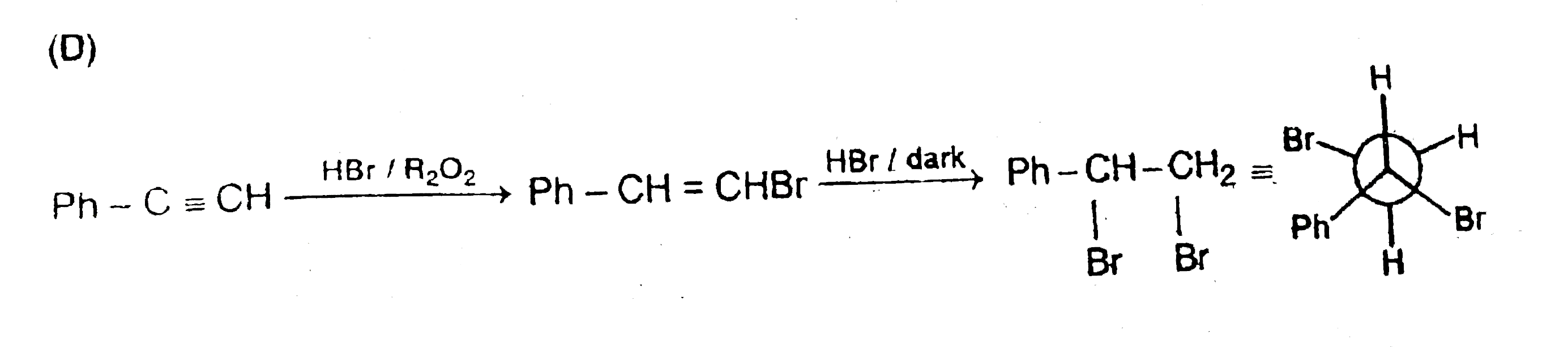
|
|
| 27909. |
The most stable conformational isomer of cyclohexane is: |
| Answer» Answer :A | |
| 27910. |
The most stable conformation of n-butane is : |
|
Answer» Skew-boat |
|
| 27911. |
The metal that cannot be produced on reduction of is oxide by aluminium is : |
|
Answer» K |
|
| 27912. |
The moststableconformation of n- butane is : |
|
Answer» SKEW boat |
|
| 27913. |
The most stable conformation of ethane is: |
|
Answer» BOAT form |
|
| 27914. |
Themetalthatcannotbe obtainedbyelectrolysisofan ofanaqueoussolution of its salt is |
|
Answer» Solution :Cabeinghighlyelectropositivecannotbeobtainedbyelectrolysisof anaqueoussolutionof itssaltbecauseCathusobtainedreactswith`H_2O ` . `Ca+2H_ 2O to Ca ( OH ) _2+H_ 2 ` |
|
| 27915. |
The most stable conformation of butane is: |
|
Answer» SKEW |
|
| 27917. |
The most stable complex ion is :- |
|
Answer» `[Fe(C_(2)O_(4))_(3)]^(3-)` |
|
| 27918. |
The most stable compound is |
|
Answer» LiF<BR>LiCl F gt Cl gt Br gt I Among alkali METALS the electropositive character increases as Lilt Na lt K lt RB lt CS Hence, LiF is the most stable compound. |
|
| 27919. |
The metal that cannot be obtained by electrolysis of the aqueous of its salts are: |
| Answer» Answer :D | |
| 27920. |
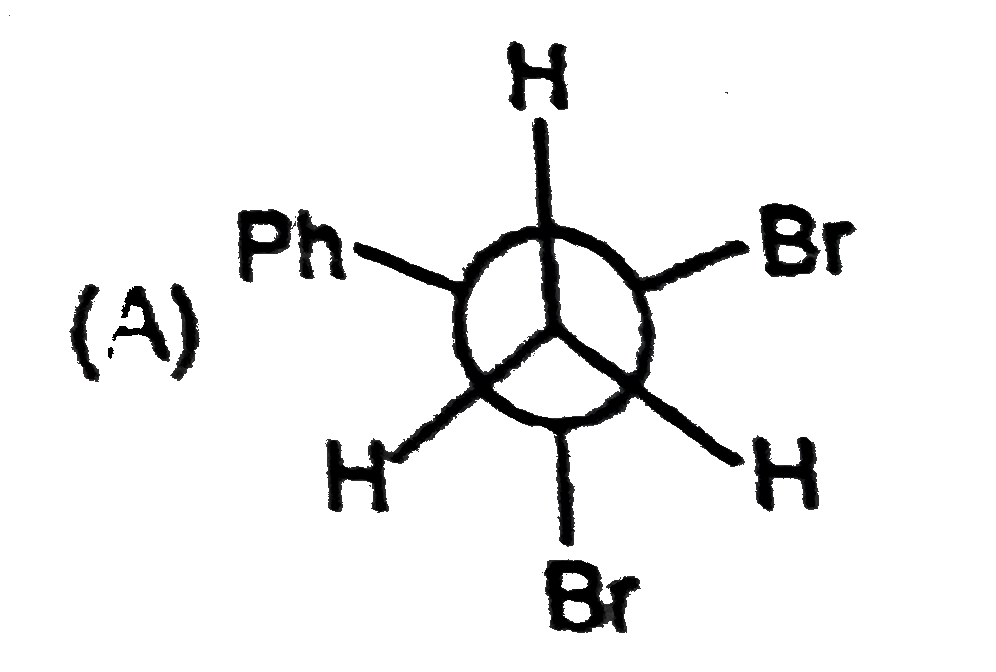
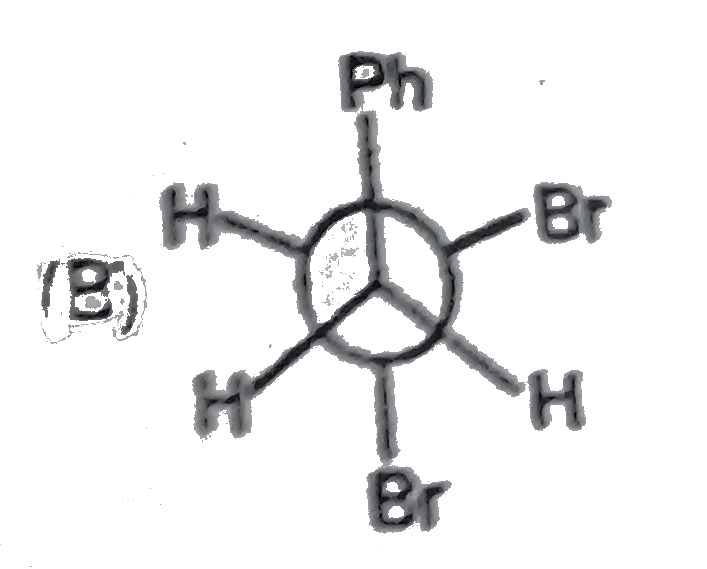
The most stable conformation of 1,2-diphynylethane is: |
|
Answer»
|
|
| 27921. |
The metal that cannot be obtained by electrolysis of the aqueous solution of their salts is: |
|
Answer» Ag |
|
| 27922. |
The most stable complex among the following is |
|
Answer» `K_(3)[Al(C_(2)O_(4))_(3)]` |
|
| 27923. |
The metal that cannot be obtained by electrolysis of an aqueous solution of its salts is |
|
Answer» Ag |
|
| 27924. |
The metal that cannot be obtained by electrolysis of an aqueous solution of its salt is |
| Answer» Answer :B | |
| 27925. |
The metal that can be obtained even by the electrolysis of its aqueous salt |
|
Answer» AL |
|
| 27926. |
The most stable complex among the following |
|
Answer» `K_(3)[Al(C_(3)O_(4))_(3)]` |
|
| 27927. |
The metal that cannot be obtained by elecrolysis of an aqueous solution of its salts is |
|
Answer» Cu |
|
| 27928. |
The most stable carbonium ion is: |
|
Answer» METHYL CARBONIUM ion |
|
| 27929. |
The most stable carbocaation is- |
|
Answer»
|
|
| 27930. |
The metal surfaces are excellent reflectors because of absorption and re-emission of light by: |
|
Answer» PROTONS in ATOM |
|
| 27932. |
The metal present in insulin is: |
|
Answer» Cu |
|
| 27933. |
The most stable and the least stable resonating structures are respectively |
|
Answer» I and IV |
|
| 27934. |
The metal present in Grignard reagent is: |
|
Answer» Ca |
|
| 27935. |
The most stable arrangement of double bonds in a polynuclear compound is the one in which the maximum number of rings possess benzenoid structure. This rule is called as |
|
Answer» HUCKEL's RULE |
|
| 27936. |
The metal present in chlorophyll is ….. And the metal present in vitamin B_(12) is …… . |
| Answer» SOLUTION :MAGNESIUM, COBALT | |
| 27937. |
The most stable allotropic form of sulphur is: |
|
Answer» RHOMBIC sulphur |
|
| 27938. |
The metal present in vitamin B_(12) is |
|
Answer» Magnesium |
|
| 27939. |
The most soluble halogen in water |
|
Answer» `F_(2)` |
|
| 27940. |
The metal presen in insulin is |
|
Answer» Cu |
|
| 27941. |
The most soluble halide in water is: |
|
Answer» `CaF_2` |
|
| 27942. |
The metal platinum in SHE is |
|
Answer» inert metal |
|
| 27943. |
The most soluble compound in water is: |
|
Answer» CuS |
|
| 27944. |
The metal oxide which decomposes on heating is/are: |
|
Answer» ZnO |
|
| 27945. |
The most satisfactory method of separating sugars from each other is : |
|
Answer» FRACTIONAL crystallisation |
|
| 27946. |
The most satisfactory method for separating sugars is : |
|
Answer» FRACTIONAL DISTILLATION |
|
| 27947. |
The metal oxide (s) which decompose on heating is (are) |
|
Answer» ZNO |
|
| 27948. |
The most reactive towards electrophilic nitration is : |
|
Answer» toluene |
|
| 27949. |
The metal oxide which cannot be reduced to metal by carbon is |
| Answer» Answer :B | |
| 27950. |
The most relative element with water among the following is: |
|
Answer» Mg |
|