Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 30601. |
The general formula of ether is : |
|
Answer» R-CHO |
|
| 30602. |
The general formula of disaccharides is …………….. |
|
Answer» `C_(N)H_(2n-2)O_(n-1)` |
|
| 30603. |
The general formula of C_nH_2nO_2 could be for open chain: |
|
Answer» CARBOXYLIC acid |
|
| 30604. |
The general formula of carboxylic acids is |
|
Answer» `C_(N) H_(2n) O_(2)` |
|
| 30605. |
The general formula of carbohydrates is: |
|
Answer» `C_nH_(2N+1)O` |
|
| 30606. |
The general formula of carbohydrates is _______. |
|
Answer» `C_(n)(H_2O)` |
|
| 30607. |
The general formula of carbohydrate is |
|
Answer» `C_(N)H_(2n+1)O` |
|
| 30608. |
The general formula of an ester, where R represents an alkyl group , is |
| Answer» Answer :C | |
| 30609. |
The general formula of amines is |
|
Answer» `C_(N)H_(2N+1)N` |
|
| 30610. |
The general formula of alkyl halides is |
|
Answer» `C_n H_(2N ) X ` |
|
| 30611. |
The general formula of aliphatic ethersis same as that of ___________. |
|
Answer» MONOHYDRIC ALCOHOLS |
|
| 30612. |
The general formula of a cycloalkane is |
|
Answer» `C_(N)H_(n)` |
|
| 30613. |
The general formula for saturated monocarboxylic acid is : |
|
Answer» `C_n H_n COOH` |
|
| 30614. |
The general formula for aliphatic ether is ________. |
|
Answer» `C_(N) H_(2N+2)O` |
|
| 30615. |
C_(n)H_(2n)O_(2) isthe general formulaof |
|
Answer» CARBOXYLIC acids |
|
| 30616. |
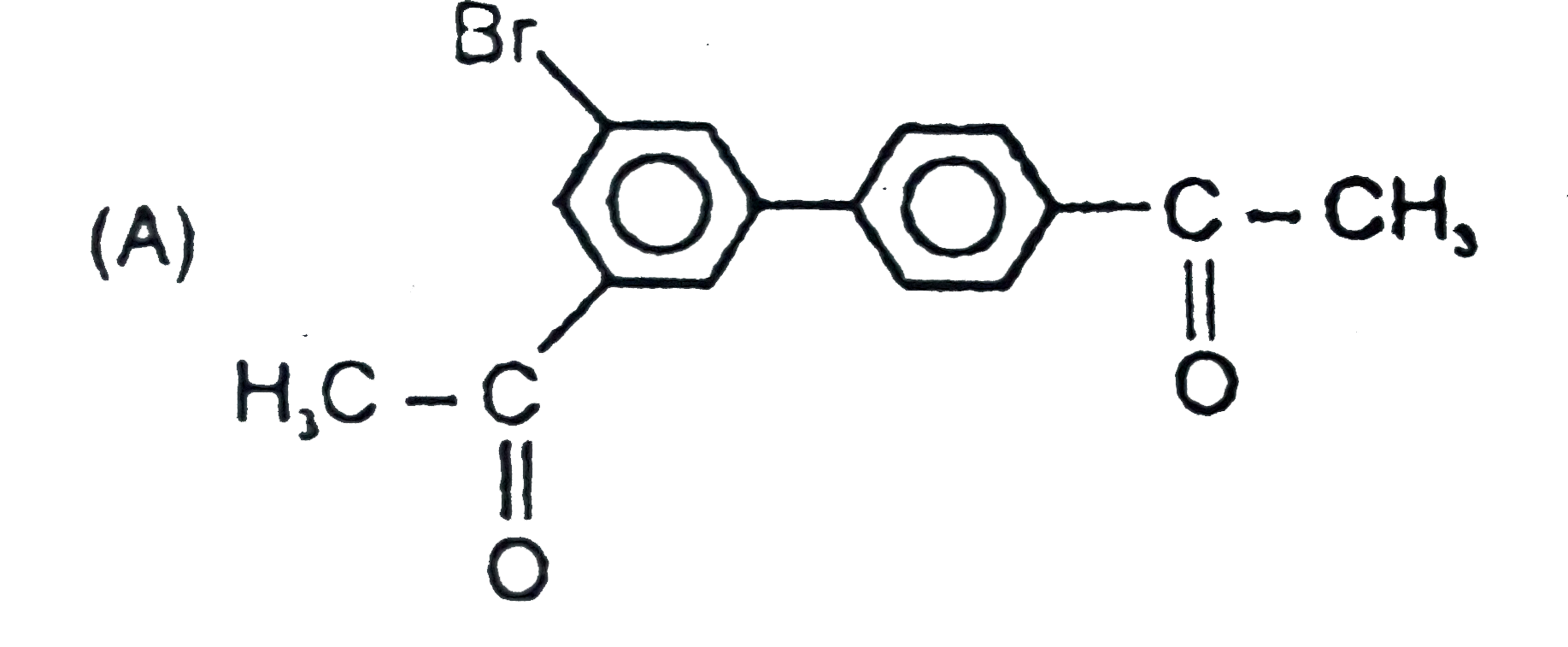
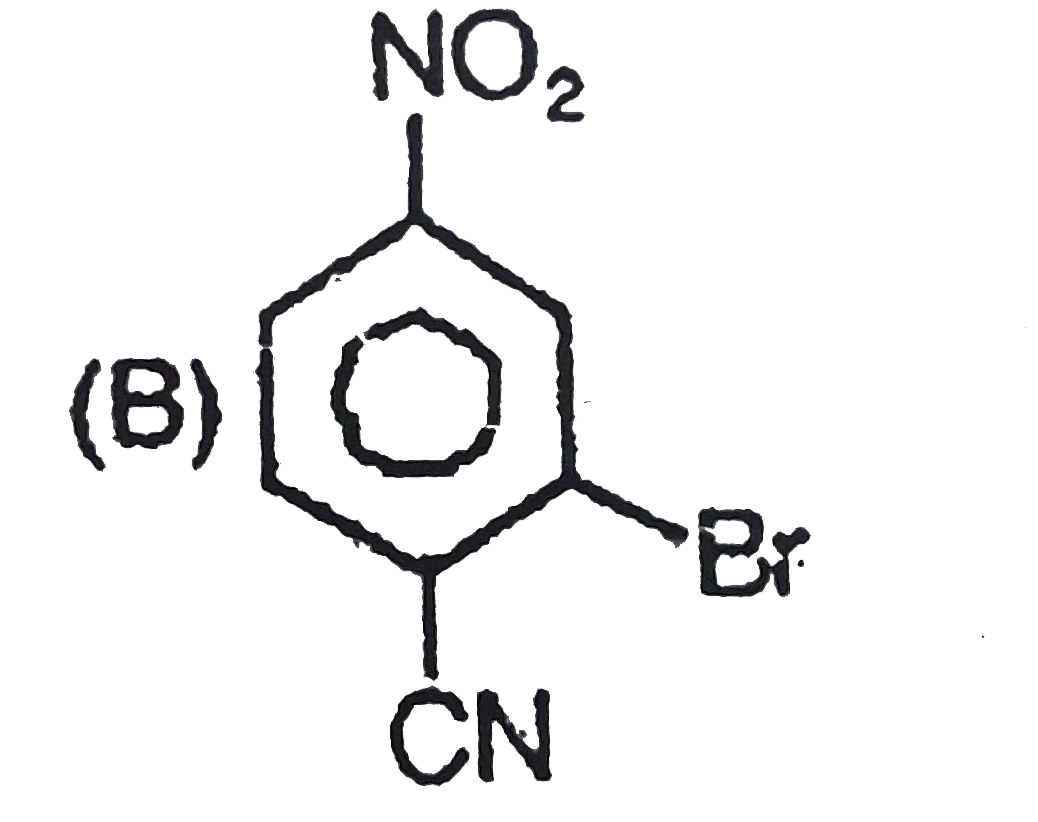
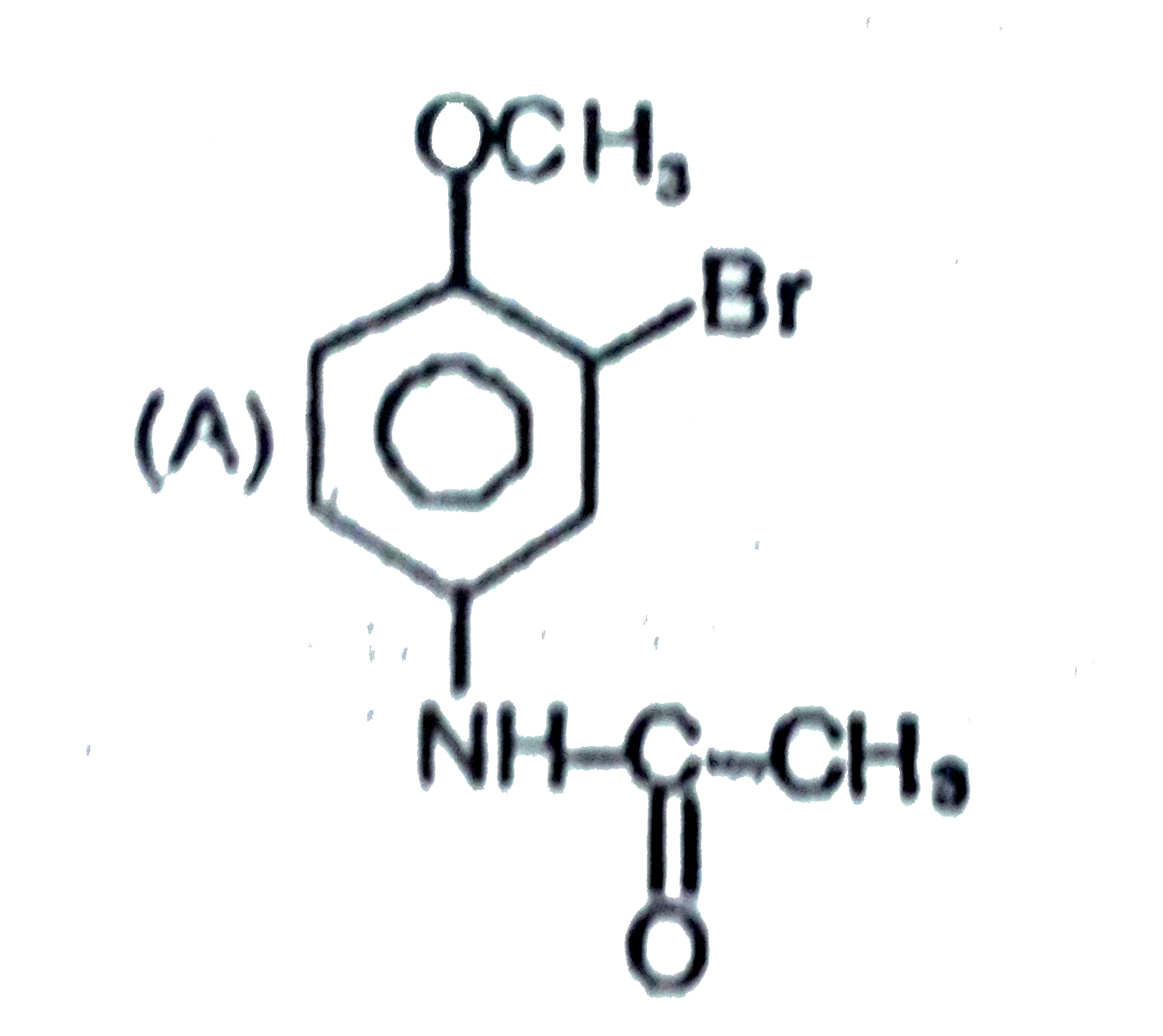
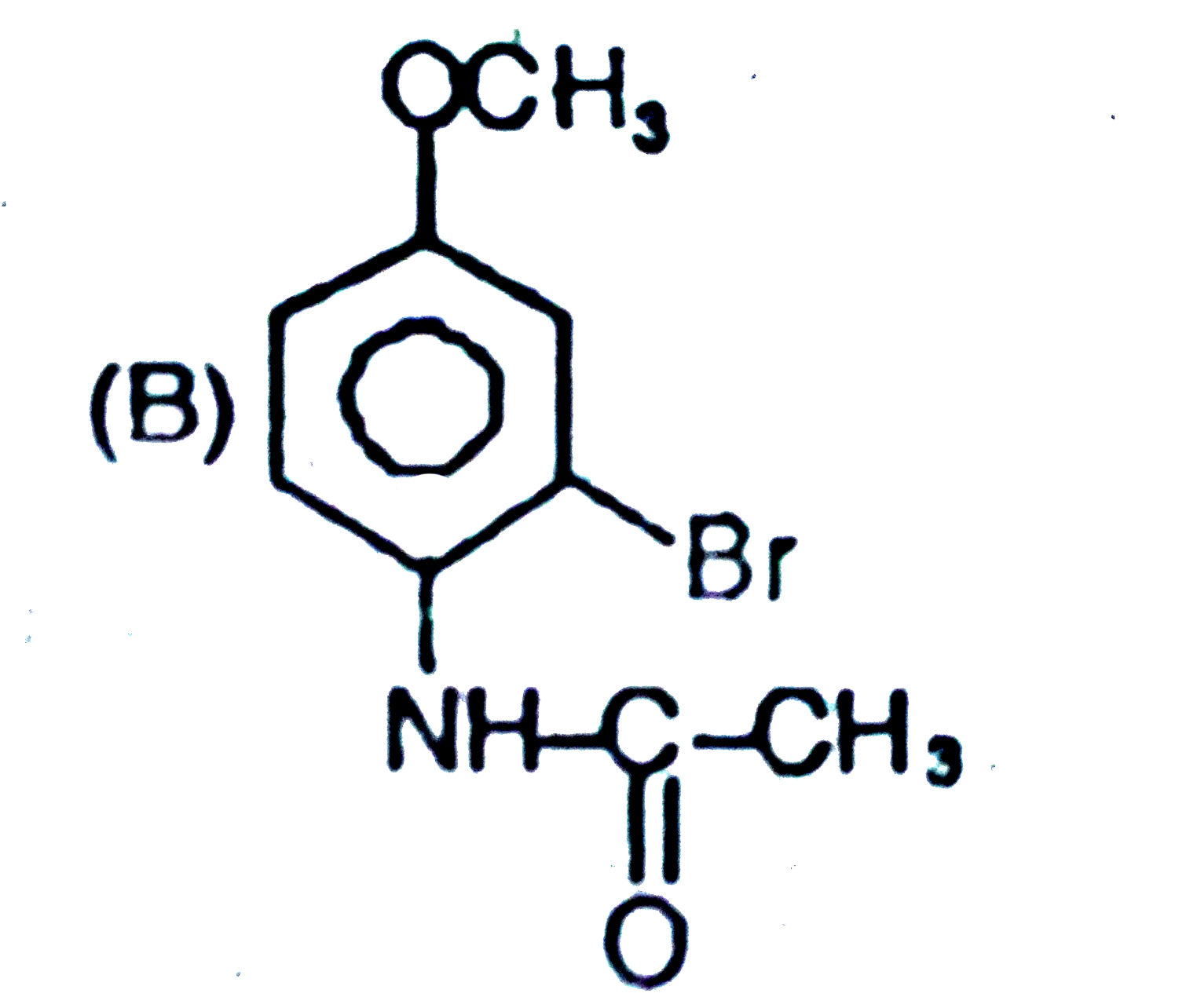
The general electrophilic substitution reaction (replacement of H. by an electrophilic which is an electron deficient species) in aromatic ring is represented as followed. An electrophilic attacks on ortho and para positions which respect to a stronger +m group are present in benzene ring and the electrophilie attacks at meta position with respect to stronger -m group if two -m groups are present in benzene ring. The product of the following reaction is |
|
Answer»
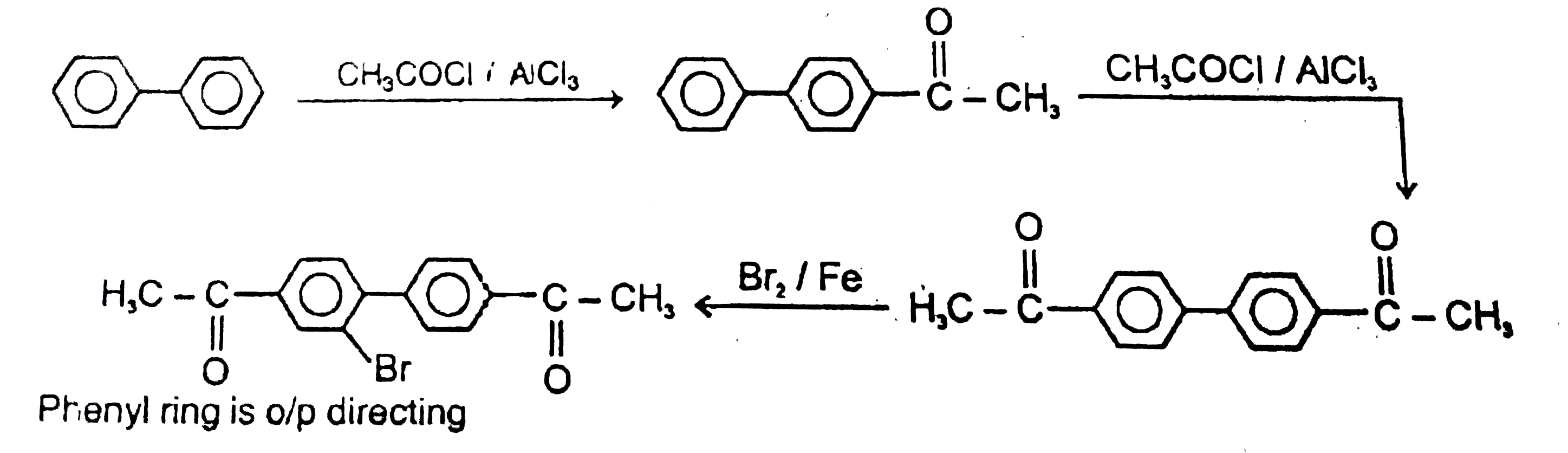
|
|
| 30617. |
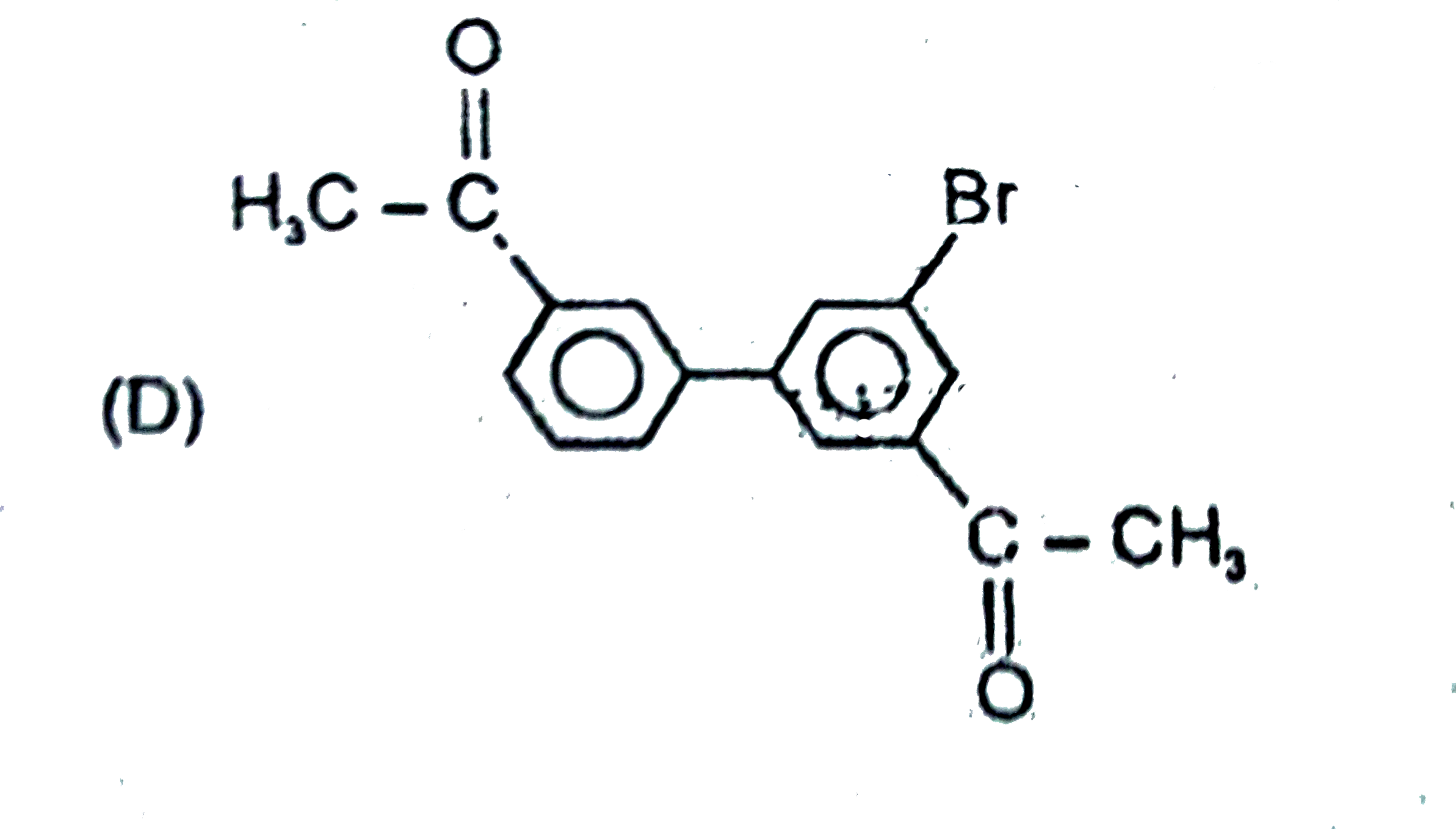
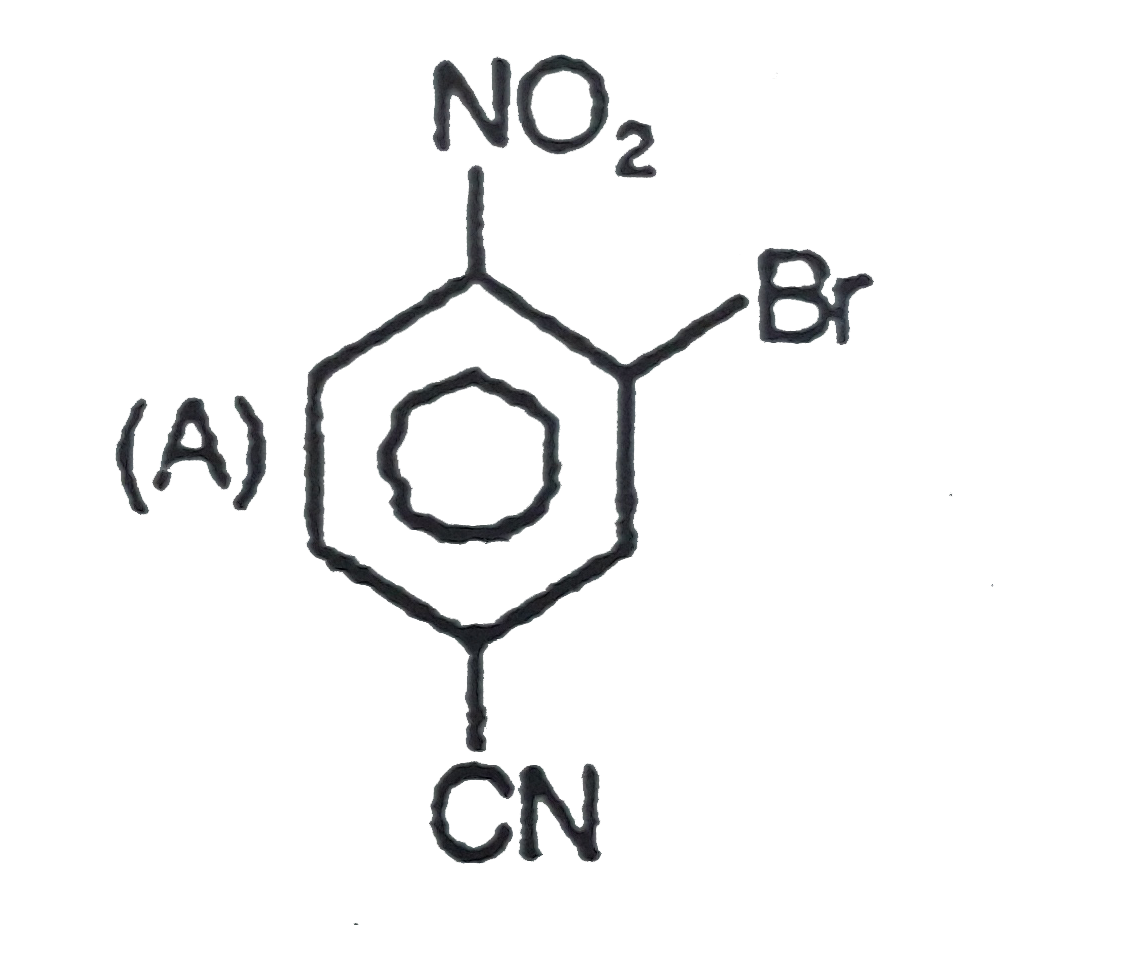
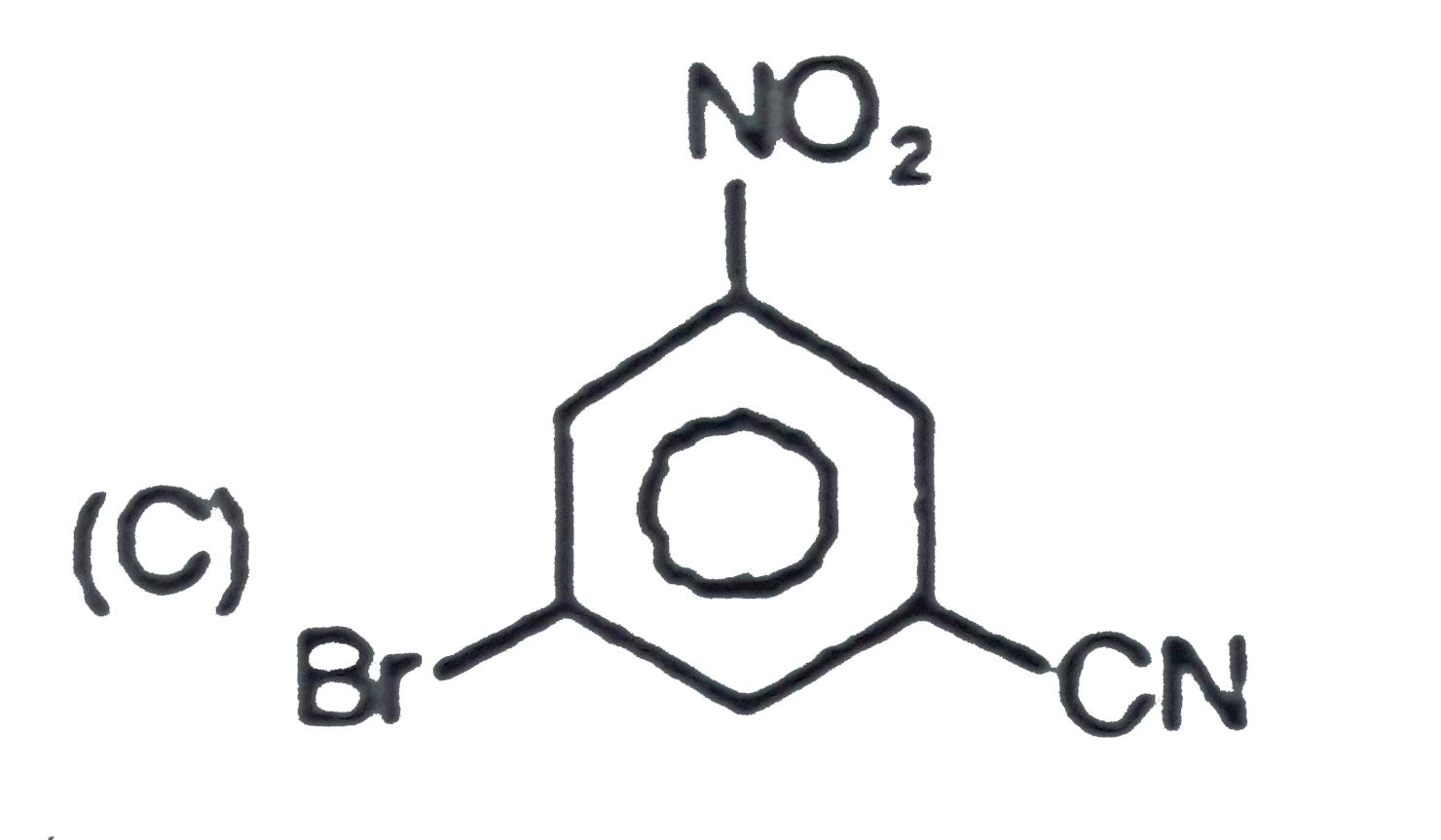
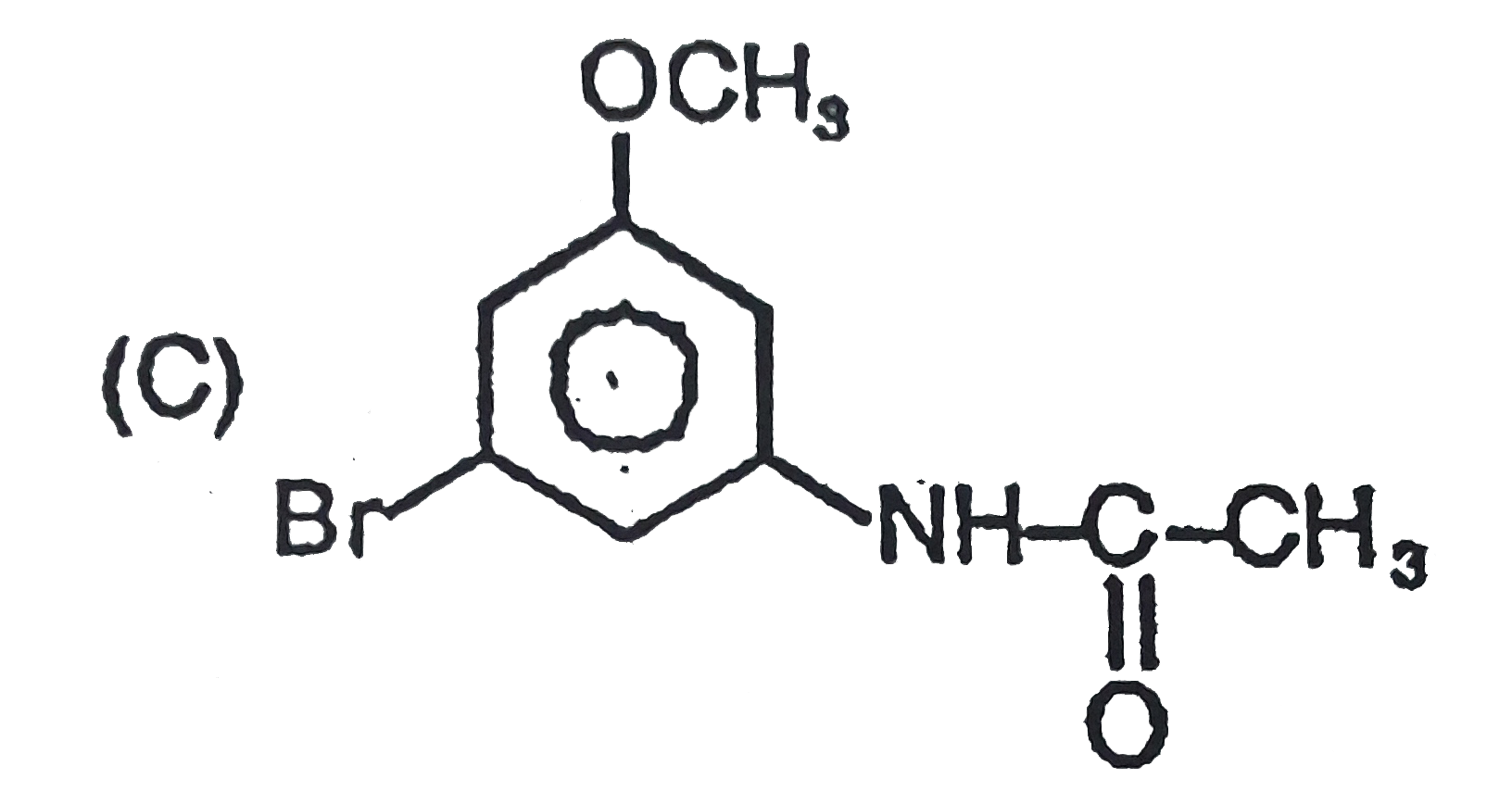
The general electrophilic substitution reaction (replacement of H. by an electrophilic which is an electron deficient species) in aromatic ring is represented as followed. An electrophilic attacks on ortho and para positions which respect to a stronger +m group are present in benzene ring and the electrophilie attacks at meta position with respect to stronger -m group if two -m groups are present in benzene ring. What will be the product when electrophile Br^(o+) attacks on |
|
Answer»
`-NO_2` will DECIDE . |
|
| 30618. |
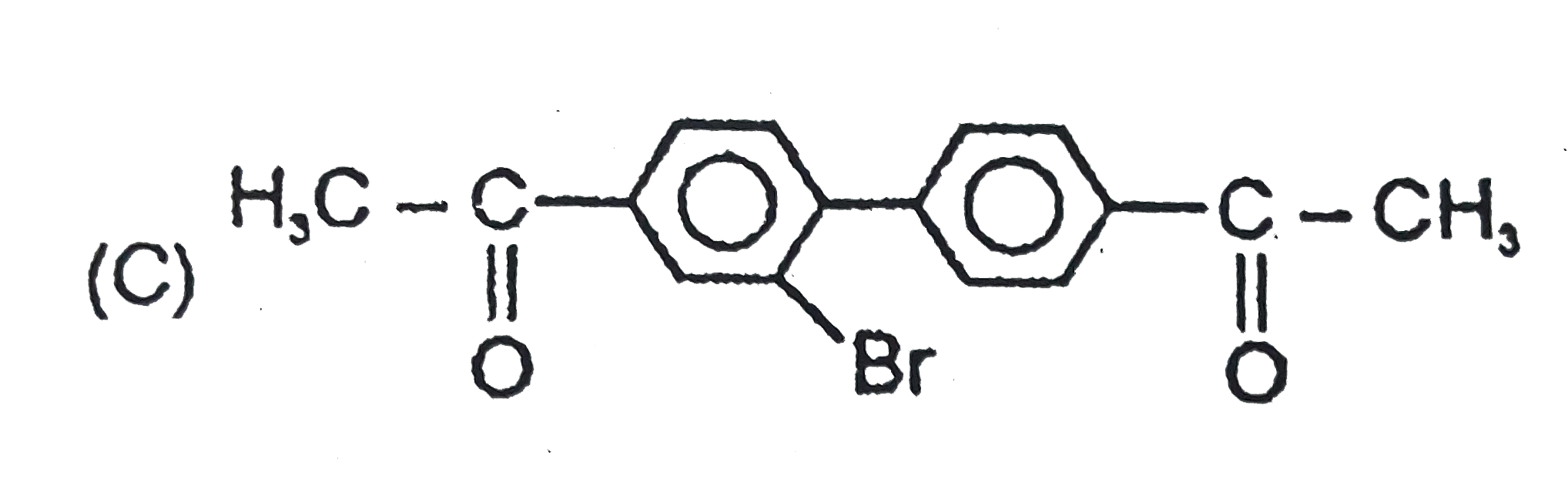
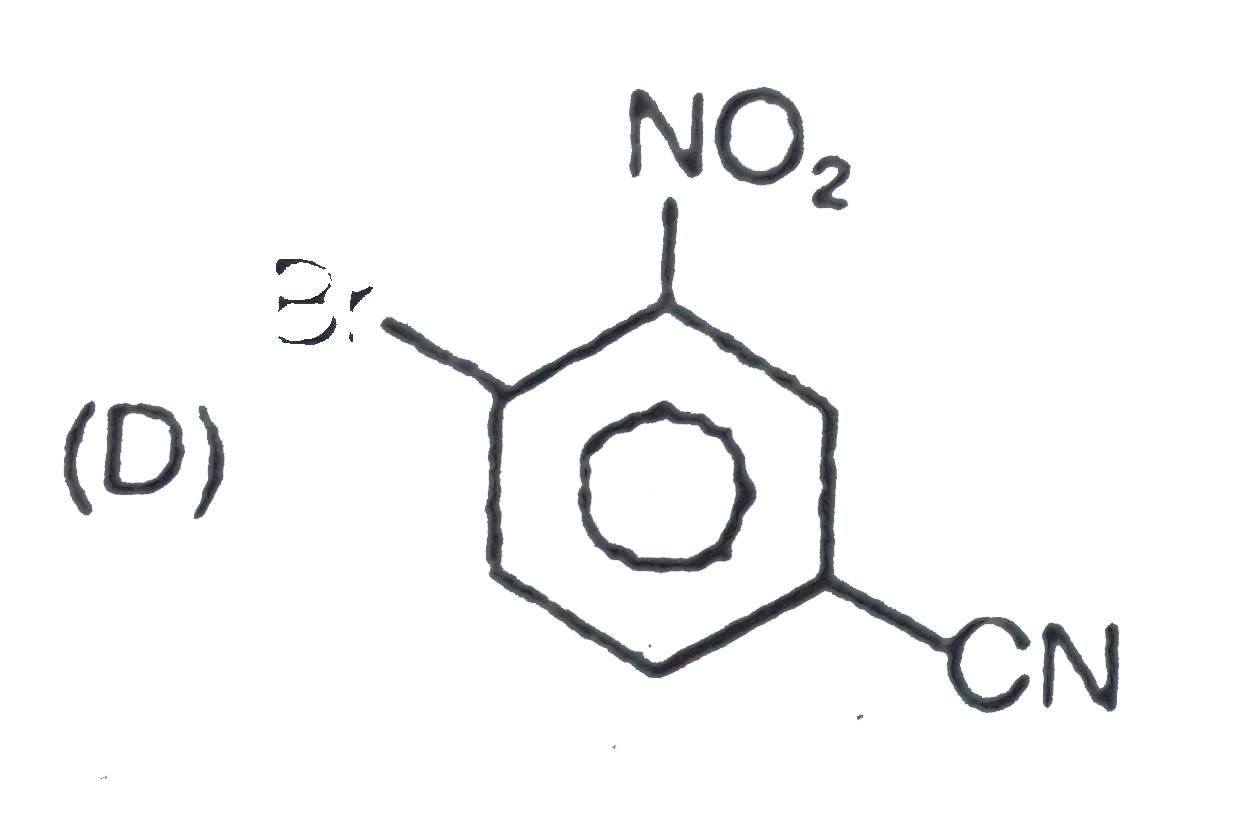
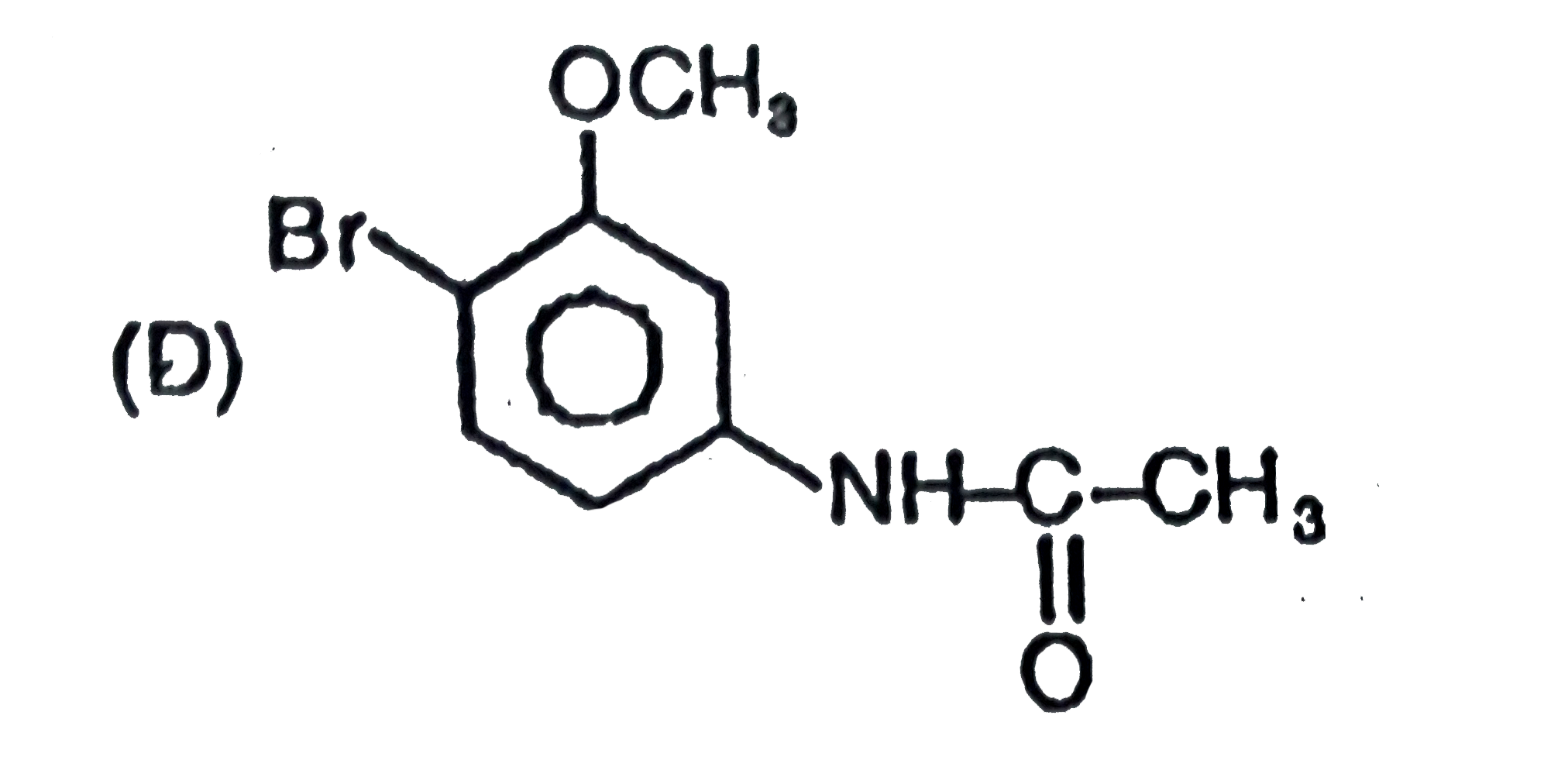
The general electrophilic substitution reaction (replacement of H. by an electrophilic which is an electron deficient species) in aromatic ring is represented as followed. An electrophilic attacks on ortho and para positions which respect to a stronger +m group are present in benzene ring and the electrophilie attacks at meta position with respect to stronger -m group if two -m groups are present in benzene ring. If electrophilic Br^(o+) attacks on the product will be : |
|
Answer»
|
|
| 30619. |
Thegeneral fomula of quaternaryammouimcompound is |
|
Answer» `R - NH_(2)` |
|
| 30620. |
The general electronic configuration of inner transition elements is "…....................". |
| Answer» SOLUTION :`(n-2)F^(1-14)(n-1)d^(0-1) NS^(2)` | |
| 30621. |
Thegeneralelectronicconfigurationof 4f - seriesof elementscan bewriteenas "_____" |
|
Answer» `[Xe] 4s^(2-14) 5D^(0-4)6S^(2)` |
|
| 30622. |
The general electronic configuration of transition elements is |
|
Answer» `(n-1)d^(1-5)` |
|
| 30623. |
The general configuration of transition element is |
|
Answer» `(n-1)d^(1-5)` |
|
| 30624. |
The gel-sol transformation on mechanical shaking and allowing to stand is called………. |
| Answer» SOLUTION :THIXOTROPY. | |
| 30625. |
The gastric juice in our stomach contains enough HCl to make the hydrogen ion concentration about 0.10 mole litre. The pH of gastric juice is |
|
Answer» 0.01 `PH = -LOG[H^(+)] = -log [10^(-2)] , pH = 2`. |
|
| 30626. |
The gas(es) which turn lime water milky is (are) |
|
Answer» `CO_(2)` |
|
| 30627. |
The gases which are responsible for photochimical smog are : |
|
Answer» OXIDES of nitrogen |
|
| 30628. |
The gases which are responsible for acid rain : |
|
Answer» OXIDES of nitrogen |
|
| 30629. |
The gases which are absorbs of IR-radiation: |
|
Answer» Oxygen |
|
| 30630. |
The gases respectively absorbed by alkaline pyrogallol and carbon tetrachloride are |
|
Answer» `O_3, CH_4` |
|
| 30631. |
The gases showing heating and cooling effect during Joule-Thomson's experiment have Joule-Thomson coeffieient: |
|
Answer» +ve and -ve respectively |
|
| 30632. |
The gases produced in the reaction, Pb(NO_(3))_(2) overset(Delta)rarr and NH_(4)NO_(3) overset(Delta)rarr are respectively |
|
Answer» `N_(2)O, NO` |
|
| 30633. |
The gases liberated during the electrolysis of aqueous solution of hydrogen fluoride |
|
Answer» `H_(2)andO_(2)` |
|
| 30634. |
The gases evolved at anode during Kolbe synthesis are |
|
Answer» `CO_(2)` |
|
| 30635. |
The gases are at absolute temperature 300K and 350 K respetivily. The ratio of average kinetic energy of their molecules is : |
|
Answer» 7:6 |
|
| 30636. |
The gases absorbed by alkaline pyrogallol and turpentine oil respectively are |
|
Answer» `O_3,CH_4` |
|
| 30637. |
The gases absorbed by alkaline pyragallol and oil of cinnamon respectively are: |
|
Answer» `O_3 , CH_4` |
|
| 30638. |
Thegaseousreactions A_(2) to 2Ais firstorderin A_(2)After12.3minutes65%of A_(2)remainsundecomposed, Howlongwillit take to decompose90%of A_(2)? Whatis thehalf- lifeof thereactions |
|
Answer» SOLUTION :(i) Timerequiredfor 90%reaction=65.8 MIN (II) Half-lifeperiod`= t_(1//2) = 19.8`min |
|
| 30639. |
The gaseous reaction A+BhArr2C+D,+Q is most favoured at |
|
Answer» LOW TEMPERATURE and high pressure |
|
| 30640. |
The gaseous reaction A+BhArr 2C+D,+Q is most favoured at |
|
Answer» low TEMPERATURE and HIGH pressure Reaction is exothermic THUS low temperature is favoured. Pressure should also be low. Hence for given reaction low temperature and low pressure is most favoured. |
|
| 30641. |
The gaseous mixture present in the 'Sun' atmosphere |
|
Answer» `AR, KR, XE` |
|
| 30642. |
The gaseous mixture used by divers for respiration is: |
|
Answer» `N_2 + O_2` MIXTURE |
|
| 30643. |
The gaseous mixture containing 2 moles of each of two ideal gases A(C_(V),m=(3)/(2)R) and B(C_(V),m=(5)/(2)R). Find out the average molar heat capacity at constant volume. |
|
Answer» 8R `=(2xx(3)/(2)R+2xx(5)/(2)R)/(2+2)=2R` |
|
| 30644. |
The gaseous decomposition reaction, A(g) rarr 2B(g) + C(g) is observed to be first order over the excess of liquid water at 25^(@)C. If is found that after 10 minutes the total pressure of system is 188 torr. The rate costant of the reaction (in hr^(-1)) is : [Given : vapour pressure of H_(2)O at 25^(@) is 28 torr ("ln" 2=0.7,"ln"3=1.1,"ln"10=2.3)] |
|
Answer» `0.02` |
|
| 30645. |
The gaseous decomposition reaction, A(g)rarr2B(g)+C(g) is observed to first order over the excess of liquid water at 25^(@)C. It is found that after 10 minutes the total pressure of system is 188 torr and after very long time it is 388 torr. The rate constant of the reaction (in hr^(-1)) is : [Given : vapour pressure of H_(2)O at 25^(@) is 28 torr (ln2=0.7, ln3=1.1, ln10=2.3)] |
|
Answer» `0.02` |
|
| 30646. |
Thegaseousenvelopearoundthe arthisknownas atmosphere. Thelowestlayerof thisis extended upto 10 kmfrom level. Thislayer is ____ |
|
Answer» Stratosphere |
|
| 30648. |
The gas with lowest boiling point is: |
|
Answer» Hydrogen  He has low boiling POINT DUE to weak INTERMOLECULAR attractive force. |
|
| 30649. |
The gas which will be most easily liquefied is |
|
Answer» `He` |
|
| 30650. |
The gas which turns mercurousnitrate paperblack is |
|
Answer» `NH_(3)` |
|