Saved Bookmarks
| 1. |
Describe the following processes: (i) Esterification (ii) Saponification (iii) Hydrogenation. |
|
Answer» Solution : (i) Esterification : Alcohols are reacted with carbonic acids in the presence of concentrated `H_2 SO_4` to form ester and this process is known as esterification. Method : Mix ethyl alochol with acetic acid in a test tube, Few drops of conc. `H_2SO_4` is added test tube is heated MILDLY in hot tube of water. Instantaneously sweet smell of ester is diffused in whole room. `CH_3COOH + C_2H_5OH to CH_3COO_2CH` (ii) Saponification : Breaking down the fats is called saponification . It is performed by heating vegetable or animal oil with 40% solution of caustic soda . Fats and base react to produce soap and glycerol. 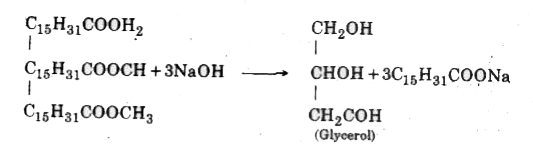 Concentrated common solution is added to crystalisesoap from water . Soap floats on the surface of water after cooling. Soap is extracted from water and desired colour and smell are added and is given desired shape . (iii) Hydrogenation : Conversion of unsaturated hydrocarbon into saturated hydrocarbons by the addition of hydrogen in the presence of Nickel or polladium as catalystis known as hydrogenation .  INDUSTRIAL APPLICATIONS : This process is used for conversion of vegetable oil into vegetable ghee . `"Vegetable Oil" + H_2 overset(Ni,476K) to "Vegetable Ghee"` Vegetableoils havedouble carbonic hand . When hydrogen gas is made to PASS through these in the presence of Nickel acting as catalyst at 473K it converts into solid fat. |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?