Saved Bookmarks
| 1. |
How will you extract aluminum from its ore? |
|
Answer» Solution :The extraction of aluminum from bauxite involves two steps: (1) Conversion of bauxite into alumina - Baeyer.s Process The conversion of bauxite into alumina involves the following steps: Bauxite ore is finely ground and heated under pressure with a solution of concentrated caustic soda solution at150 ° C to obtain sodium meta aluminate.On diluting sodium meta aluminate with water, a precipitate of aluminum hydroxide is formed The precipitate is filtered, washed, dried and ignited at `1000^(@)C` to get alumina. `2Al(OH)underset(Delta)overset(1000^(@)C)toAl_(2)O_(3) + 3H_(2)O` (ii) Electrolytic reduction of alumina - Hall.s Process Aluminum is produced by the electrolytic electrolytic process of manufacturing aluminum reduction of fused alumina`(Al_(2),O_(3))` in the electrolytic cell.Cathode: Iron tank linked with graphite Anode: A bunch of graphite rods suspended in molten electrolyte. Electrolyte: PURE alumina + molten CRYOLITE carbon cathode lining + fluorspar (fluorspar lowers the fusion TEMPERATURE of electrolyte) Temperature: 900 - 950 ° C Voltage used: 5-6 V Overall reaction: `2 Al_(2)O_(3)rightarrow4Al+3O_(2)uparrow`Aluminum is DEPOSITED at the cathode and oxygen gas is liberated at the anode.Oxygen combines with graphite to form `CO_(2)` 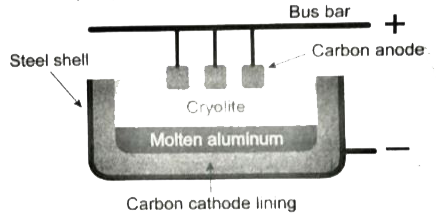
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?