Saved Bookmarks
| 1. |
(ii) Ag_(s) +HCI_(I) to AgCI darr + H_(2) uarr |
|
Answer» Solution :Step 1 :Rewrite the given equation as it is ` Ag_(s) + HCI_(I) to AgCI darr + H_(2)` Step 2 :Writethe number of atoms of each ELEMENT in theunbalanced equation on bothsides of equations. 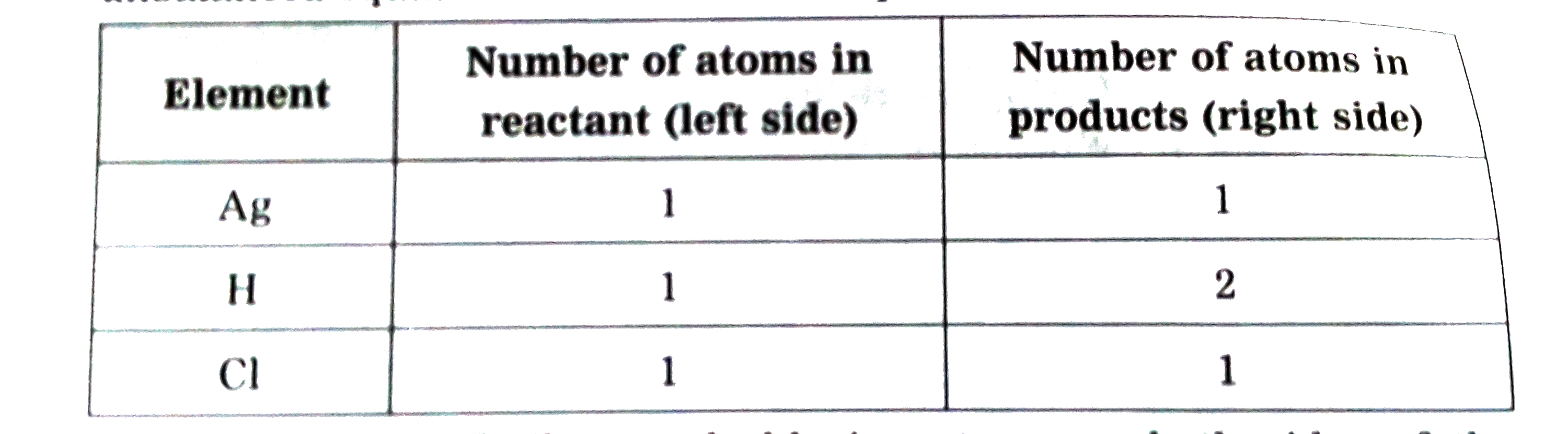 The number of silver and chlorine atoms on bothsides of the equation are same, therefore, EQUALISE the number of hydrogen atoms. Step 3:To balance the number of hydrogen atoms . 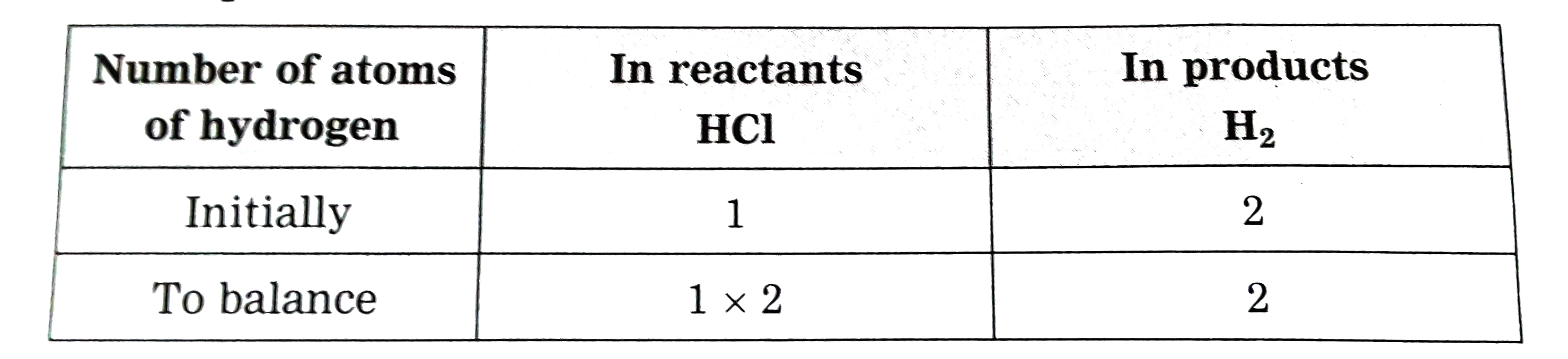 To equalise the number of hydrogen atoms, we use 2 as the factor in the product HCI, now the unbalanced equation become ` Ag + 2HCI to AgCI + H_(2)` Step 4 : To balancethe number of chlorine atoms :  To equalise the number of chlorine atoms, we use 2 as the factor in the product , AgCI , now the unbalanced equation becomes ` Ag + 2HCI to 2AgCI + H_(2)` Now count the atoms of each element on both sides of the equation, there are LESS number of silver atoms in the reactant. Now equalise the silver atoms, the balanced equation becomes. `2Ag + 2HCI to 2AgCI + H_(2)` Now indicate the physcial states of the reactantsand products ` 2Ag_((s)) + 2HCI_(4(AQ)) to 2AgCI darr + H_(2) uarr ` |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the reason for the twinkling of stars ?
- The change in magnetic field lines in a coil is the cause of induced electric current in it.Name the underlying phenomenon.
- Say True or False.The mass of the Earth is 6.4xx10^(6)kg.
- Whichof the follwing property of a proton can changewhileit moves freelyin a mageticfield ? (There may be more thanone correct answer).
- When an object is placed infront of a spherical mirror at a distance 30 cm, the magnification is -1. Illustrate the conclusions.
- Does the frequency of sound waes depend on the medium in which it travels?
- The relation between N (no. of molecules), P,V. & T is ...........
- What is magnification of a lens ?
- A person is said to be colour blind if he/ she has deficiency of rod shaped cells in retina of his eyes.
- "___________" prepares the 'Red List' that contains the names of endangered species from different countries.