InterviewSolution
Saved Bookmarks
| 1. |
(iii) H_(2)SO_(4(aq)) + NaOH_((aq))to Na_(2)SO_(4(aq))+ H_(2)O_((I)) |
|
Answer» Solution :Rewrite the given equation as it is ` H_(2)SO_(4(aq)) + NaOH_((aq)) to Na_(2)SO_(4(aq)) +H_(2)O_((l))` STEP 2 : Write the number of atoms of each element in the unbalanced equation on both sides of the equation . 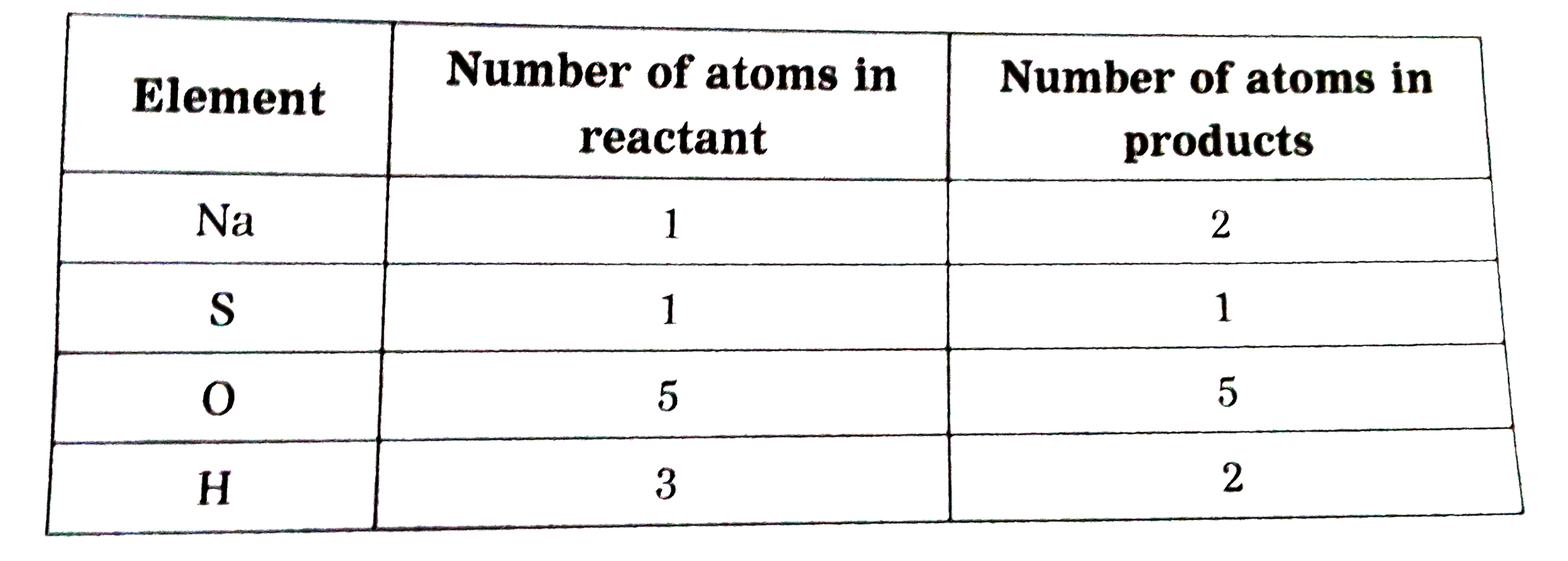 The number of OXYGEN atoms involved in different compounds on both sides (reactants and PRODUCTS) are equal. THEREFORE, balance the number of atoms of the second element, sodium. Step 3 :To balance the number of sodium atoms :  To equalise the number of sodium atoms, we use 2 as the factor of NaOH in the reactants. Now, the partly balanced equation becomes as follows : ` H_(2)SO_(4) + 2NaOH to Na_(2)SO_(4) +H_(2)O` Step 4 : Now, balance the number of hydrogen atoms :  To equalise the number of hydrogen atoms, we use 2 as the factor of ` H_(2)O` in the products. The equation then becomes `H_(2)SO_(4) + 2NaOH to Na_(2)SO_(4) + 2H_(2)O` Now, count the atoms of each element on both sides of the equation. The number of atoms on both sides are equal. Hence, the balanced equation is ` H_(2)SO_(4(aq)) + 2NaOH_((aq)) to Na_(2)SO_(4) + 2H_(2)O_((l))` |
|