Saved Bookmarks
| 1. |
The students of class 9 of City International school were attending an audio-visual session on the collinsion theory of chemical kinetics. When they were viewing the slides given below, their instructor asked them to guess which of the following could be the effective collision and justify their answer. |
Answer» Solution :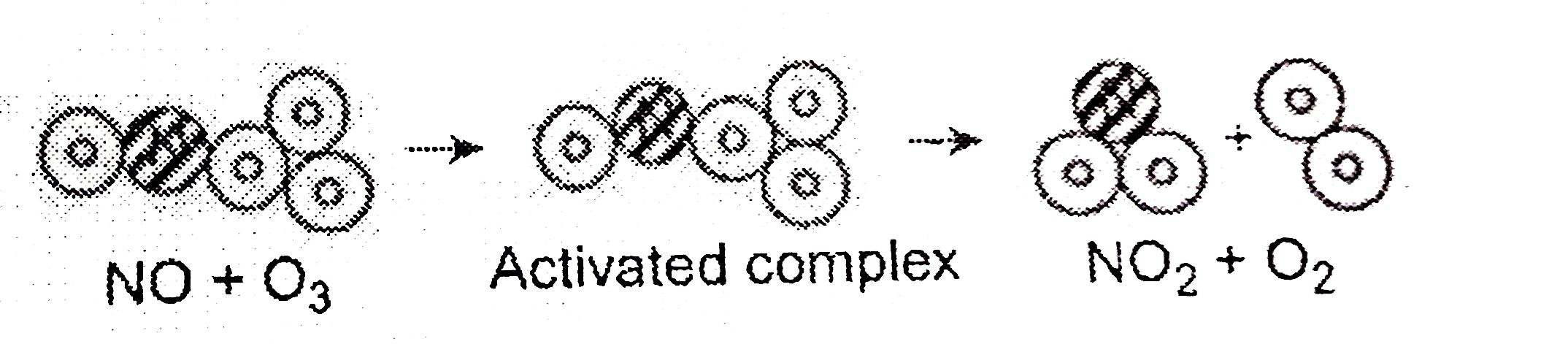 The collisions REPRESENTED by FIGURE (a) are effective collisions. Thecollisions with threshold energy result in a reaction. APART from this, the reactants which collide should have a proper orientation. In figure (b), the nitrogen atom of NO is not near enough to make a bond with any of the near enough to make a bond with any of the oxygen atoms of ozone. In figure (a), the NO molecule collides with `O_(3)` where the direction in which both the reactants collide is PERFECT enough to result in a CHEMICAL reaction. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Two ice blocks of 10 g each are placed in 2 L distilled water at 273 K. One of theice blocks is made up of distilled water. What will you observe if the ambient temperature is also 273 K ? Give reasons to support your observation.
- Which separation techniques will you apply for the separation of the following ? (a) Sodium chloride from its solution in water. (b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride. ( c) Small pieces of metal in the engine oil of a car. (d) Different pigments from an extract of flower petals. (e) Butter from curd. (f) Oil from water. (g) Tea leaves from tea. (h) Iron pins from sand. (i) Wheat grains from husk. (j) Fine mud particles suspended in water.
- The teacher instructed three students 'A', 'B' and 'C'respectively to prepare a 50% (mass by volume) solution of sodium hydroxide (NaOH). 'A' dissolved 50g of NaoH in 100 mL of water. 'B' dissolved 50g of NaOH in 100g of water while 'C' dissolved 50g of NaOH in water to make 100 mL of solution. Which one of them has made the desired solution and why?
- What are impure substance? Give example.
- Why are cotton clothes prefferred in summer ?
- What is the effect of heat on the rate of diffusion?
- What is the mass of 1 mole of nitrogen atoms ?
- What is superphosphate of lime ?
- CHARACTERISTICS OF PARTICLES OF MATTER
- The separation of denser particles from lighter particles done by rotation at high speed is calls _______