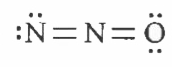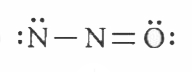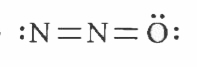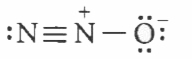Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 20201. |
Waxes are long chain compounds belongingto the class: |
|
Answer» Acids |
|
| 20202. |
Which of the following is the correct electron dot structure of N_(2)O molecule? |
|
Answer»
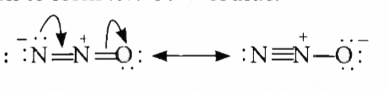
|
|
| 20204. |
Which of the following have lowest boiling point ? |
|
Answer» He |
|
| 20205. |
When H_3BO_3 is strongly heated, __________ is finallyobtained. |
| Answer» SOLUTION :`B_2O_3` (BORON TRIOXIDE) | |
| 20206. |
When a buffer solution of CH_(3)COOH and CH_(3)COONaisdiluted withwater |
|
Answer» `CH_(3)COO^(-)`ION concentration increases |
|
| 20207. |
The units of rate constant for first order equation. |
|
Answer» `s^-1` |
|
| 20208. |
The solubility product of iron (III) hydroxide is 1.6xx10^(-39). If x is the solubilityof iron (III) hydroxide, then which of the following expressions can be used to calculate x ? |
|
Answer» `K_(sp)=x^(4)` `K_(sp)=(x)(3x)^(3)=27 x^(4)`. |
|
| 20209. |
Which products are expected from the disproportionation reaction of hypochlorous acid? |
|
Answer» `HCIO_(3) and CI_(2)O` |
|
| 20210. |
What are the different types of complexes ? |
|
Answer» Solution :There are three types of complexes:(i) Cationic complexes: These are the complexes in which the complex ion carries a NET positive charge. Example: `[NI(NH_3)_6]^(2+),[Cr(NH_3)_6]^(3+)` ETC, (ii) (ii) Anionic complexes: These are the complexes in which the complex ion carries a net negative charge. Example: `[FE(CN)_6]^(4-),[Fe(CN_6)]^(3-)` etc. (iii) Neutral complexes: These are. the complexes which do not yield opposite charge ions in SOLUTIONS. Example: `[Fe(CO_4)],[CO,Cl_C(NH_3)]` etc. |
|
| 20211. |
What is meant by auto ionisation of water? |
Answer» Solution :Pure WATER has a little tendency to dissociate i.e., ONE water molecule DONATES a proton to ANOTHER water molecule. This is know as AUTO ionisation of water. 
|
|
| 20212. |
which of the following series belong to the visible region of emission spectra? |
|
Answer» Lyman |
|
| 20213. |
Which of the following is an ionic compound ? |
|
Answer» ONE molecular orbital |
|
| 20214. |
Which of the following can act as bridging ligands? |
|
Answer» `OH^(-)` |
|
| 20215. |
Which equation relates the cell potential and the concentration of the species involved in an electro chemical reaction? |
|
Answer» Henderson equation |
|
| 20216. |
which of the following anions are easilyremoved from aqueous solution by precipitation ? |
|
Answer» `CI^(Θ)` |
|
| 20217. |
Which monomer is used to preparation orlon ? |
|
Answer» `CF_(2)=CF_(2)` |
|
| 20218. |
The toal number of protons in 10g of calcium carbonate is |
|
Answer» `3.015xx10^(24)` |
|
| 20219. |
Which can adsorb large volumes of hydrogen gas ? |
|
Answer» Colloidal solution of palladium |
|
| 20220. |
The scientist Sheele first prepared Cl_(2)by heating Conc. HCl with |
|
Answer» SNO |
|
| 20221. |
The reduction of R-COOH to R-CH_(2)OH can be effected by : |
|
Answer» sodium/alcohol |
|
| 20222. |
The separation of landthanoids in ionexchange method is based on : |
|
Answer» BASICITY of the hydroxides |
|
| 20223. |
The standard cell potential for the cell is , Zn|Zn^(2+)(1M)||Cu^(2+)(1M)|Cu [E^@ for Zn^(2+)//Zn=-0.76V, E^@ for Cu^(2+)//Cu=+0.34V] |
|
Answer» `-0.76+0.34=-0.42V` |
|
| 20224. |
Which monosaccharide glucose units are present in sucrose and lactose ? |
|
Answer» Solution :`ALPHA`-D GLUCOSE and `beta`-D fructose are present in SUCROSE. `beta`-D GALACTOSE and `alpha`-D glucose are present in lactose. |
|
| 20225. |
When PCl_5 reacts with sulphuric acid sulphuryl chloride (SO_2Cl_2) is formed as the final product. This shows that sulphuric acid: |
|
Answer» Is a diabasic acid |
|
| 20226. |
Which of the following reagents reacts in same manner with HCHO, CH_3CHO, CH_3COCH_3: |
|
Answer» HCN |
|
| 20227. |
Which one of the following pairs of substances could be used for the preparation of copper (II) sulphate in the laboratory? |
|
Answer» Copper and dilute SULPHURIC acid |
|
| 20228. |
Using a conductivity cell with "0.9 cm"^(-1) cell constant, the conductance was observed to be 2.5 xx 10^(-3)" mho for 0.07 M KCl" solution. What is the specific conductance of the solution ? |
| Answer» SOLUTION :`2.25xx10^(-3)" MHO. CM"^(-1)` | |
| 20229. |
To produce difference between freezing point and boiling point of a solution by 105.0^(@)C, how much sucrose should be dissolved in 100 gm of water ?(K_(f)=1.86^(@)C, kg mol^(-1) " and " K_(b)=0.151^(@)Ckg mol^(-1)) |
|
Answer» 72 gm `= 100+K_(b).m` Freezing point `(T_(f))=0-Delta T_(f)=K_(f).m` `therefore T_(b)-T_(f)=(100+K_(b).m)-(-K_(f).m)` `105=100+0.151 m+1.86 m` `2.37 m =5` `m = (5)/(2.37)=2.11` So, mass of sucrose to dissolve in 100 gm WATER is, `= (2.11xx342xx100)/(1000)=72` gm. |
|
| 20230. |
Which one of the following transition element has maximum oxidation state ? |
|
Answer» MANGANESE |
|
| 20231. |
What is EDTA (Ethylendiamine tetraacetic add) ? Give one use. |
| Answer» Solution :EDTA is a HEXADENTATE LIGAND. It HELPS to estimate HARDNESS of water. | |
| 20232. |
Which of the following is an appropriate set of eactants for the preparation of 1-methoxy-4 nitrobenzene. Why? |
| Answer» SOLUTION :Reactants in set(II) is suitable and appropriate for the preparation of the product l-methoxy-4. NITROBENZENE but not those of set I. This is because aryl halides are very less REACTIVE in NUCLEOPHILIE substitution reaction | |
| 20233. |
Which of the following aqueous solutions has the highest freezing point ? |
|
Answer» 0.1 molal `AI_(2)(SO_(4))_(3)` Thus, lower the value of I, lower is `DeltaT_(f)` and higher is the FREEZING point of the solution. Since 'I' is LEAST (2) for `NH_(4)CI, DeltaT_(f) "for" NH_(4)CI` isalso minimum. freezing point of NH_(4)CI solution is the maximuum. |
|
| 20234. |
Whichofthefollowingreactionsoccur duringcalcination ? |
|
Answer» ` CA CO _3toCa O+CO_ 2 ` |
|
| 20235. |
Which type of drugs come under antimicrobial drugs ? |
| Answer» SOLUTION :ANTISEPTICS, DISINFECTANTS, ANTIBIOTICS and sulpha DRUGS. | |
| 20236. |
Which one is least ionic? |
|
Answer» AgCl |
|
| 20237. |
The standard Gibbs energy change at 300 K for the reaction 2A hArr B +C is 2494.2J. At a given time, the composition of the reaction mixture is [A]=(1)/(2), [B]=2 and [C]=(1)/(2). The reaction proceeds in the : [R=8.314 J//K//"mol", e=2.718] |
|
Answer» forward DIRECTION because `Q lt K_(e )` `2A hArr B+C` `R=8.314 J//K"mol"`. e= 2.718 `[A]=(1)/(2), [B]=2, [C]=(1)/(2)` `Q=([B][C])/([A]^(2))=(2xx1//2)/((1)/(2))^(2)=4` `Delta G^(@)= - 2.303 " RT LOG " K_(e)` `2494.2J= - 2.303xx(8.314 J//K//"mol")xx(300K)log K_(e )` `implies " log " K_(e )= -(2494.2 J)/(2.303xx8.314 J//K//"mol" xx300K)` `implies log K_(e )= -0.4341` `K_(e )=0.37` `Q gt K_(e )` |
|
| 20238. |
Which of the structures given below are chiral ? |
|
Answer» I,II,III |
|
| 20239. |
which of thefollowinglanthanoid ions is diamagnetic ? |
|
Answer» `EU^(2+)` |
|
| 20241. |
What are the factors on which cell potential depends? |
| Answer» Solution :The CELL VOLTAGE DEPENDS on the nature of the electrodes, the concentration of the ELECTROLYTES and the temperature at which the cell is operated. | |
| 20242. |
Whate are the examples of first order reaction ? |
|
Answer» Solution :(i) Decompostion of DINITROGEN pentoxide `N_2O_(5(G))rarr2NO_(2(g))+1//2O_(2(g))` (II) Decompostion of thionylchoride `SO_2Cl_(2(g))rarrSO_(2(g))+Cl_(2(g))` (iii) Decomposition of `H_2O_2` in aqueous solution `H_2O_(2_((aq)))rarrH_2O_((l))+1//2O_(2(g))` (iv) Isomerisation of CYCLOPROPANE to propene |
|
| 20243. |
Write Howorth structure of ''Lactose''. |
Answer» SOLUTION :
|
|
| 20244. |
Which of the following compounds shows the presence of intramolecular hydrogen bond |
|
Answer» Concentrated acetic ACID |
|
| 20245. |
Which of the following compounds has isopropyl group |
|
Answer» 2,2,3,3-tetramethylpentane |
|
| 20246. |
What volume of water must be added to 500 ml NaOH to make 2% (w/v) NaOH |
|
Answer» `5000 mL` `= 3 xx 40` `= 120 gm` `2% ((W)/(v)) NaOH` `(120)/(V) xx 100 = 2` `V = 6000 mL` Volume added `= (6000 - 500) mL` `= 5500 mL` |
|
| 20247. |
Which of the following represents decreasing order of reactivity of given organic compounds towards nitration. (i) Terephthalic (ii) Toluene (iii) p-Toluic acid (iv) p-Xylene (v) m-Xylene |
|
Answer» `IV gt Vgt II gt III gt I` |
|
| 20248. |
Which of the M^(4+) ion has more tendency to gain single electron ? |
|
Answer» `CE ^(4+)` |
|
| 20249. |
Which of the following complexes follow Sidgwick EAN rule? |
|
Answer» `FE(eta^(5)-C_(5)H_(5))_(2)]` |
|
| 20250. |
Which of the following is not an example of +E effect: |
|
Answer» Hydrohalognation of Propene |
|