Explore topic-wise InterviewSolutions in Current Affairs.
This section includes 7 InterviewSolutions, each offering curated multiple-choice questions to sharpen your Current Affairs knowledge and support exam preparation. Choose a topic below to get started.
| 1. |
Small water droplets associated with dust particles to form larger aggregates called ______ |
|
Answer» |
|
| 3. |
Shakti along with his parents was going to the reilway station by an auto to catch a train. Shakti's father was recollecting his childhood days and said that in those days, autos were run with petrol only. His mother added that diesel was used to run autos in later days. Shakthi said that nowadays, autos are preferably run with CNG, Shakti questioned his father regarding the advantage of CNG over petrol and diesel. What was his father's explanation ? |
| Answer» Solution :CNG does not produce any HARMFUL GASES and THUS it reduces the maintenance cost and it a clean fuel. While petrol and DIESEL CONTAIN N and S which upon combustion produce `NO_(2)" and "SO_(2)` respectively. These lead to acid rain. Hence CNG can be a substitute for petrol and diesel. | |
| 4. |
Separation of sawdust from water, can be carried out by the following steps given below. Arrange them in a proper sequence. (a) The mixture is poured gently in to the filter cone and collected into another beaker which is called filtrate. (b) A mixture of saw dust and water is taken in a beaker (c ) A filter paper is folded in the form of a cone and fitted into a funnel by moistening it with few drops of water (d) solid retained on the filter paper is called residue. |
|
Answer» cabd (ii) A filter PAPER is FOLDED in the FORM of a cone and fitted into a funnel by moistening it with few drops of water (iii) The mixture is poured gently in to the filter cone and collected into another beaker which is called filtrate. (iv) solid RETAINED on the filter paper is called RESIDUE. |
|
| 5. |
Sand and saw dust are mixed with water. Name the techniques that can separate sand and saw dust from water. |
| Answer» SOLUTION :As the DENSITY of sand is more than saw DUST, it settles down and saw dust floats on water. Sand can be separated by SEDIMENTATION and decantation. Saw dust can be separated by filtration. | |
| 6. |
'Sand and clay which are abundantly available in theearth's crust have significant roles in the comfort zone of modern life''. Comment on this statement. |
|
Answer»
|
|
| 7. |
Salt used in purification of water is |
|
Answer» potash alum |
|
| 8. |
Salt solutions are good conductors of electricity. This is due to the presence of _______ in solution state. |
|
Answer» mobile electrons |
|
| 9. |
Salt is added to pure water to increase its electrical conductivity. |
|
Answer» |
|
| 10. |
Saline water contains large amounts of dissolved salts. |
|
Answer» |
|
| 11. |
Rusting of iron is an example of both physical and chemical change. |
|
Answer» Rusting of iron is a chemical change in which iron GETS CORRODED in HUMID AIR . |
|
| 12. |
Role of nitre in the manufacture of gun powder is ______ . |
|
Answer» to supply OXYGEN |
|
| 13. |
Regeneration of anion exchange resin is carried out by passing |
|
Answer» sodium HYDROXIDE SOLUTION |
|
| 14. |
Rate of evaporation of water |
|
Answer» is more in COASTAL area than in non coatal area. |
|
| 15. |
Pyrex or borosil glass is used for making laboratory apparatus whereas crown glass in used in themanufacture of optical instruments. Give reason. |
| Answer» SOLUTION :PYREX or borosil glass consists of borax which reduces theexpandability of glass and provides heat resistance whereas of red lead in crown glass increases THEDENSITY of glass to a GREAT extent making it useful for optical INSTRUMENTS. | |
| 16. |
Pure nitric acid on longstanding turns to yellow due to the formation of |
|
Answer» `O_(2), NO "and" H_(2)O` `4HNO_(3) RARR 4NO_(2) + 2H_(2)O + O_(2)` |
|
| 17. |
Pure HNO_(3) is colourless, howerver, it gradually becomes yellow on standing due to _______ . |
|
Answer» the decomposition of `HNO_(3)` and formation of NO. `4HNO_(3) rarr 2H_(2)O + 4NO_(2) + O_(2)` |
|
| 18. |
Property responsible for spreading of fragrance of flower is ________ . |
|
Answer» compressibility of gas/vapour |
|
| 19. |
Property exploited in the usage of perfumes is _______ . |
|
Answer» compressibility of GASES |
|
| 20. |
Preparation of salt from sea water involves |
|
Answer» EVAPORATION |
|
| 21. |
Predict the valencies of the elements with atomic numbers 7 , 15 , 16 , 18 , 19 and justifyAlso give the formulae of hydrides formed by the above elements if any. |
Answer» SOLUTION :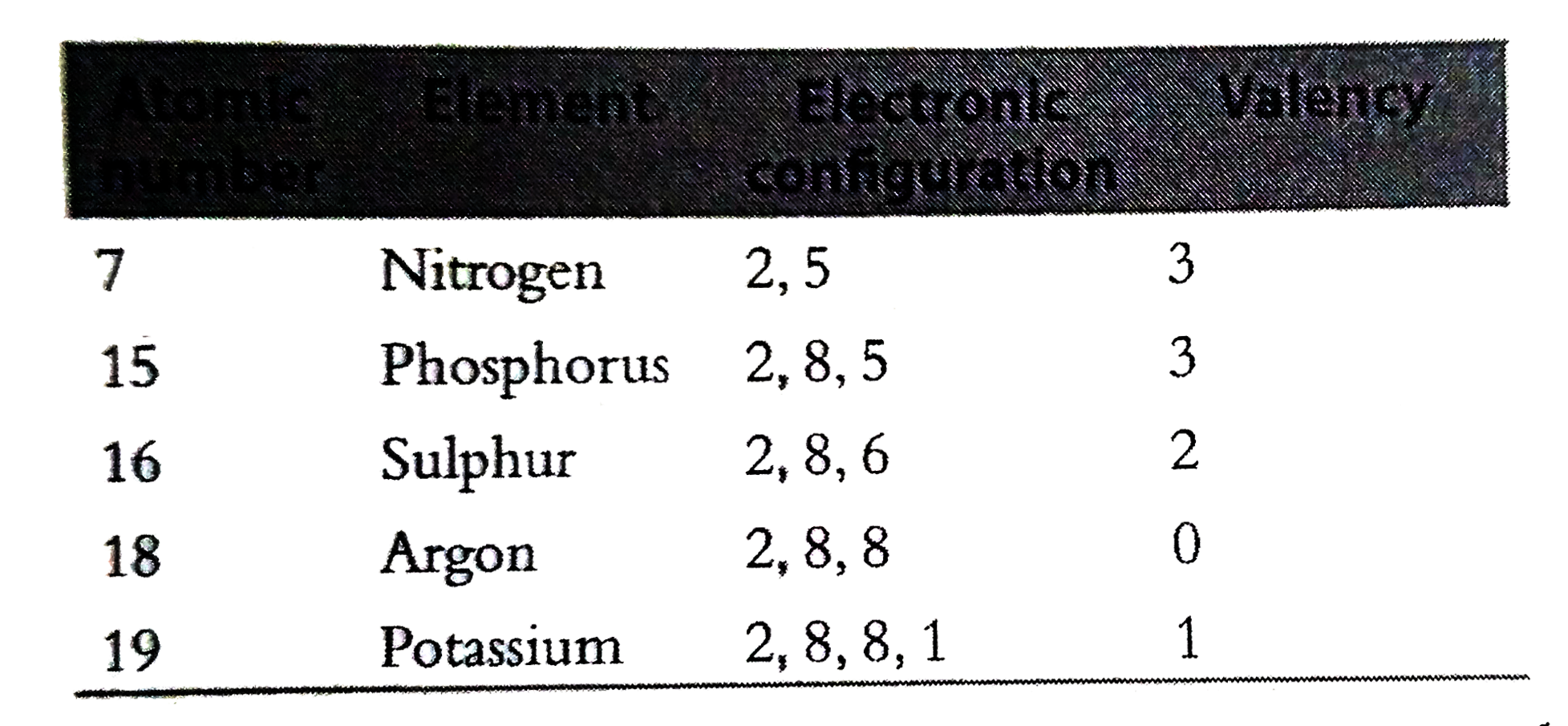 Since N andP need three more electrons to ATTAIN octet , their valencies are 3. Similarly , the valencies of other elements with atomic NUMBER 16 , 18 , 19 are 2 , 0 and 1 respectively. Except argon , all other elements forms hydrides. Phosphorus`- PH_(3)`, Potassium- KH Nitrogen`NH_(3)` SULPHUR`- H_(2)S` Argon does not from any COMPOUND |
|
| 22. |
Potassium chlorate on strong heating gives potassium chloride and oxygen . Whattype of reaction is this? |
| Answer» SOLUTION :It is DECOMPOSITION REACTION. | |
| 23. |
Platinum is used as a catalyst. |
|
Answer» REASON : Platinumused as a catalyst for most of the REACTIONS that take PLACE in gaseouse state. |
|
| 25. |
Pick out the correct statement (s) among the following. |
|
Answer» An ALLOY is a homogenous mixture of two more METALS. |
|
| 26. |
Permanent hardness is removed by Clark's method. |
| Answer» Solution :CLARK's method is USED for the removal of TEMPORARY HARDNESS. | |
| 27. |
Percentage of water in animals varies from ______ to ______. |
|
Answer» |
|
| 28. |
People used to apply a coating of sodium silicate on eggs for the purpose of preservation. Justify. |
| Answer» SOLUTION :Sodium or potassium SILICATE is used as a protective covering for EGGS. When egg is dipped in solution of sodium silicate inwater a layer of sodium silicate forms on the surface of egg which is very impervious to water, air and heat.As a result bacterial growth PREVENTED in egg so that it can be preserved for relatively longer periods of time. | |
| 29. |
Ozone present in …………plays a role in protecting the life on surface from harmful UV rays. |
|
Answer» Troposphere |
|
| 31. |
Oxygen is neutral towards litmus. |
|
Answer» |
|
| 32. |
One molecule of H_(3) PO_(4) is treated with one molecule of NaOH, two molecues of NaOH and three molecules of NaOH separately. Comment on the nature of products formed and write their names. |
|
Answer» SOLUTION :`H_(3)PO_(4) + NaOH rarr underset("Acidic salt")(NaH_(2)PO_(4) + H_(2)O)` `H_(3)PO_(4) + 2NaOH rarr underset("Acidic salt")(Na_(2)HPO_(4) + 2H_(2)O` `H_(3)PO_(4) + 3NaOH rarr underset("NEUTRAL salt")(Na_(3)PO_(4) + 3H_(2)O)` `NaH_(2)PO_(4) rarr` Sodium dihydrogen phosphate `Na_(2)HPO_(4) rarr` DISODIUM hydrogen phosphate `Na_(3)PO_(4) rarr` Sodium phosphate |
|
| 33. |
One molecule of an 'ic' acid of a nonmetal having 5 electrons in valence shell reacts with a molecule of base to form a salt 'X'. The base corresponds to the metal with one electron in valence shell. If the salt so formed can react with the same base in 1 : 2 ratio, predict the formula of the salt 'X'. |
|
Answer» `K_(2)SO_(4)` |
|
| 34. |
On passing electricity through acidulated water, the gaseous products obtained are collected in two separate test tubes A and B. The volume of the gas collected in test tube A is double the volume of gas collected in test tube B. Identify the two gases in test tube A and B. |
|
Answer» Hydrogen and oxygen |
|
| 35. |
On passing electricity through acidulated water, the gaseous products obtained are collected in two separate test tubes A and B. The volume of the gas collected in test tube A is double the volume of gas collected in test tube B. Identify the two gases in test tubes A and B respectively. |
|
Answer» Hydrogen and OXYGEN |
|
| 36. |
Odd one among the following with respect to the strength of acids is |
|
Answer» PHOSPHORIC acid |
|
| 37. |
O_(2)" and "CO_(2) prepared in the laboratory can be collected by ………. |
|
Answer» downward displacement of WATER and downward displacement of air respectively |
|
| 38. |
Number of atoms on either side of a chemical equation is balanced by keeping appropriate coefficients and not by changing the subscripts of the element in the formulae . Justify . |
|
Answer» Solution :If subscripts are changed, the molecular FORMULA the is COMPOSITION is changed. Hence the number of atoms on either side of a CHEMICAL equatoin is balanced by keeping appropriate coefficients. |
|
| 39. |
Non-metals usually exist in _______ state. |
|
Answer» A non-metal GENERALLY EXISTS in the gaseous STATE. |
|
| 40. |
Non-metals are generally non-malleable and non-ductile. |
|
Answer» REASON : Non-metals are GENERALLY nonmalleable and nonductile |
|
| 41. |
Noble gases are chemically _________ . |
|
Answer» NOBLE GASES are CHEMICALLY inert. |
|
| 42. |
Nitrogen dilutes the activity of ………. In atmosphere. |
|
Answer» Ar |
|
| 43. |
Nitre is the common name of sodium nitrate. |
|
Answer» |
|
| 44. |
Nitre is the common name of __________. |
|
Answer» |
|
| 45. |
Naturally occurring diamonds are sometimes foundin different colours. Give reasons. |
| Answer» Solution :Naturally occurring DIAMONDS are ASSOCIATED with some IMPURITIES. These impurities have CHARACTERISTIC colours and RETAIN these colours in diamonds, hence naturally occurring diamonds are found to be in different colours. | |
| 46. |
Nascent hydrogen is a strong ………… agent. (reducing/oxidizing) |
|
Answer» |
|
| 48. |
Name two metals which find application in ayurvedic medicine. |
| Answer» Solution :Gold and silver METALS FIND APPLICATION in AYURVEDIC medicine. | |
| 49. |
Name two metals that can exhibit variable valency .Also give thenames of redicals. |
|
Answer» Solution :COPPER and Iron `{:("Copper :","Cuprous "Cu^(+)),(,"Cupric "Cu^(2+)),("Iron:","Ferrous "FE^(+2)),(,"FERRIC "Fe^(+3)):}` |
|
| 50. |
Name two metal carbontes from which carbon dioxide can not be prepared by heating. |
| Answer» SOLUTION :CARBONDIOXIDE cannot be PREPARED by heating SODIUM carbonate and POTASSIUM carbonate. | |