Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 1101. |
Radiochemistry deals with ………………. |
| Answer» Answer :C | |
| 1102. |
Raagav brings his lunch every day to school in a plastic container which has resin code number 5. The container is made of _______ |
|
Answer» Polystyrene |
|
| 1103. |
Protrons and neutrons have almost the same mass. |
|
Answer» |
|
| 1105. |
Protons and Neutrons |
Answer» SOLUTION :
|
|
| 1106. |
Proton was discovered by |
|
Answer» RUTHERFORD |
|
| 1108. |
Protium, Deutrium and Tritium are isotopes of |
|
Answer» Rhodium |
|
| 1109. |
Process of simple distillation can be used to separate the constituents from a liquid mixture differing the their boiling points by 25^(@)C or more. However, fractional distillation is effective if the difference in boiling points is less than 25^(@)C. How will you explain this ? |
| Answer» Solution :In simple distillation, only the low boiling liquid will DISTIL while high boiling liquid will remain in the distillation flask. THUS, separation can be done if the difference in the boiling points of the liquids is `25^(@)C` or more. In the second case, both the liquids will distil simultaneously. The distillate will contain the fractions of both the liquids. If a fractionating column is used, the VAPOURS of high boiling liquid will also rise into the column along with the low boiling liquid. But they will condense first releasing energy (called latent HEAT of condensation) and fall back in the distillation flask as a liquid. This energy will be absorbed by the vapours of the low boiling liquid which will remain in the vapour state. It will get distilled while the high boiling liquid unable to get distilled, remain in the distillation flask only. In this way, separation can be done. Thus, the role of fractionating column is to put obstructions in the path of the vapours of the liquids that are rising upwards. | |
| 1110. |
Pressure has no influence on the following equilibrium. Justify. N_(2(g))+O_(2(g)) hArr 2 NO_((g)) |
| Answer» | |
| 1111. |
Predict the position of the element which form the largest cation and the smallest anion in the modern periodic table |
|
Answer» Solution :(i) cations are smaller than their corresponding atoms and anions are larger that their corrsponding atoms (ii) trend of atomic size ALONG a period and down a group (iii) POSITION of the largest atom which can FORM CATION (iv) position of the smallest atom which can form ANON |
|
| 1112. |
Predict the position and properites like metallic /non metallic character and oxidizing / reduciing capacity of an element with atomic number 35 in the periodic table |
| Answer» SOLUTION :ELEMENT with atomic number 35 is bromine and its electoniic configuration is 2,8,18,7. Bromine belons to VIIA(17) group (halogens) .DUE to this electonic configuration bormine has strong electron affinity hence it is a reducing agent .Since bromine has SEVEN valence electron it is a NON metal | |
| 1113. |
Predict the effect of pressure on the following equilibria : (i) 2Cu(NO_(3))_(2(s)) hArr 2CuO_((s))+4NO_(2(g))+O_(2(g)) (ii) C_((s))+CO_(2(g)) hArr 2CO_((g)) (iii) I_(2(g))+5F_(2(g)) hArr 2IF_(5(g)) (iv) FeO_((s))+CO_((g)) hArr Fe_((s))+CO_(2(g)) |
|
Answer» SOLUTION :(i) Le Chatelier's principle (ii) comparision of the number of moles of gaseous REACTANTS and products (iii) effect of number of moles on volume (iv) effect of PRESSURE on volume (v) shift of REACTION to DECREASE the effect of pressure |
|
| 1114. |
Pragya tested the solubility of three different substances at different temperature and collected the data as given below (result are given in the following table, as grams of substance dissolved in 100 gms of water to form a saturated solution. (a)What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K ? (b ) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools ? Explain. (c )Find the solubility of each salt at 293 K which salt has the highest solubility at this temperature ? (d )What is the effect of change of temperature on the solubility of a salt ? |
|
Answer» Solution :At 313 K, 62g of potassium NITRATE dissolved in 100G of water. So to produce a saturated solution of potassium nitrate in50 g of water we need - `(62)/(100) xx 50 = 31` g of potassium nitrate (b )Some soluble potassium chloride will SEPARATED out in the form of crystal at room temperature because the solubility of potassium chloride will decrease. (c )(i) Solubility of potassium nitrate at 293 K is 32g (ii) Solubility of sodium chloride at 293 K is 36g (iii) Solubility of potassium chlorde at 293 K is 35G (iv) Solubility of Ammonium Chloride at 293 K is 37g The solubility of Ammonium Chloride is highest at this temperature. (d ) The Solublity of salt increases with the increase in temperature |
|
| 1115. |
Pratap is a student of class IX in a city school. He has recently studied the writting of formulae of ionic compounds in the class. Pratap has made some placards for a science quiz showing the symbols of some elements and some ions but forgot to write the electric charges on the ions. Moreover, the placards made by Pratap got mixed up as shown below : Based on these placards, the chemistry teacher, Mr. Suri, asked Pratap to answer the following question : (a) Choose the symbol of the element from among the placards which can form divalent and trivalentcations. Name this element. Also write the symbols of these cations along with their charges (b) Choose the symbol of a divalent anion from among the given placards. what is the name of this anion. Also write the symbol of this anion alongwith its charge (c) Work out the formula of the ionic compound formed between the divalent cation and divalent anion described above. Also name the compound formed (d) Work out the formula of the ionic compound formed between the trivalent cation and divalent anion described above. Also name the compound formed (e) What values are displayed by Pratap in this episode ? |
|
Answer» Solution :(a) The positively charged ions are called cation. The symbol of element which can form divalent cations. (valency 2+ ions) and trivalent cations (valency 3+ ions) is Fe. The name of the this element is iron. The symbols of two cations formed by iron element are `Fe^(2+)` [ferrous ion or iron (II) ion] and `Fe^(3+)` ion [ferric ion or iron (III)ion] (b) The negatively charged ions are called anions. The symbol of the divalent anion (valency 2-ion) is `SO_(4)`. The name of this anion is sulphate ion. The symbol of sulphate ion ALONG with its charge is `SO_(4)^(2-)` (c) The divalent cation (or valency 2+ ion) is `Fe^(2+)` and the divalent anion (valency 2- ion) is `SO_(4)^(2-)`. The formula of compound formed from these ions can be worked out as follows : 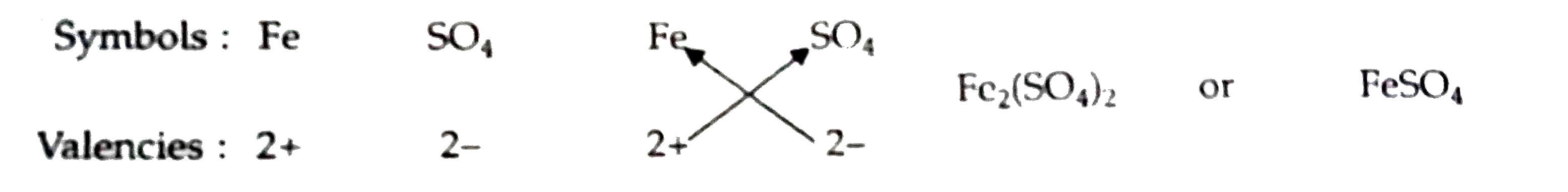 THUS, the formula of compound formed is `FeSO_(4)`. It is ferrous sulphate or iron (II) sulphate (d) The trivalent cation (or valency 3+ ion) is `Fe^(+)` and the divalent anion (or valency 2- ion) is `SO_(4)^(2-)`. The formula of compound formed from these ions can be worked out as follows : 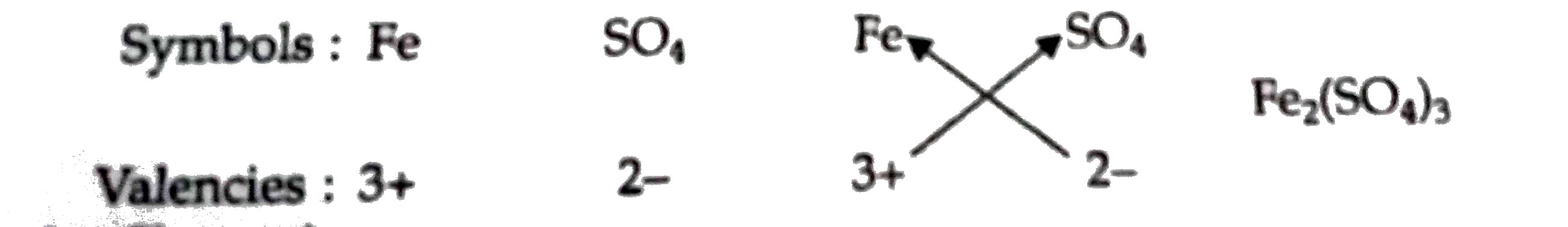 Thus, the formula of compound formed is `Fe_(2)(SO_(4))_(3)`. It is ferric sulphate or iron (III) sulphate (e) The VALUES displayed by Pratap in this episode are (i) Knowledge of cations (positive ions) and anions (negative ions), and their valencies, and (ii) ABILITY to use this knowledge in solving PROBLEMS. |
|
| 1116. |
Pragyatested the solubility of three differenttemperaturesand collectedthe data as given below ( resultsare given inthe followingtable , as grams ofsubstancesdissolvedin 100gmof water to forma saturatedsolution )(a) What a massof Potassium nitratewould be neededto producea saturatedsolutionof potassiumNitratein 50gmof waterat 313k? ( b)Pragya makesa saturatedsolution of potassiumcholride in waterar 353Kand leaves the solutionto coolat room temperature. What would sheobserveas the solutioncools ? Explain . (c)Find the Solubilityof the eachsalt at 293KWhich salthas the Highestsolubilityat thistemperatures? (d)What is theeffectof change the temperatureon the solubility of a salt ? |
|
Answer» Solution : (a)`KNO_(3)` neededfor Saturatedsolutionat 313K , which isabout 62gm = `("620" xx" 50.)/100 = 31gm` (B)KCL on coolingto get Saturatedsolution . ( c)Solubility at 293K (i)PotassiumNitrate=32 gm (ii)SODIUM Cholride = 36gm (III)Potassium Chloride =35gm (iv) Ammonium Chloride = 37gm Ammonium Cholride , solubility is more thanat this tempearture ( solubility increaseswithincrease in temperature ) (d)Rate of Solubility increses withincrease in Temperature. |
|
| 1117. |
Prachi took 50 mL of water in two beakers at room temperature and added sodium chloride to one beaker while sugar to the other, till no more solute would dissolve. Then she heated the contents of the beakers and added more solutes in them. (a) Will the amount of salt and sugar that can be dissolved in water at given temperature same ? (b) What will you expect to happen if she cools the contents of the beakers ? Justify your answer. |
| Answer» SOLUTION :(a) No, they will be different. Actually SODIUM chloride (salt) is a crystalline solid while sugar is a molecular solid. They DISSOLVE to different extent. Sodium chloride is more soluble in water compared to sugar at a given temperature. (b) Upon cooling, solid solute will slowly start separating from the solution. In general, the SOLUBILITY of a solid in a liquid increases with the RISE in temperature and decreases as the temperature is lowered. | |
| 1118. |
Potential energy of the products is ___ than reactants in an endothermic reaction. |
|
Answer» |
|
| 1119. |
Polyethene |
|
Answer» polyenthene |
|
| 1120. |
Plastics made of Polycarbonate (PC) and Acrylonitrile Butadiene Styrene (ABS) are made of resin code ________ |
|
Answer» 2 |
|
| 1121. |
Plaster of Paris should be stored in a moisture proof container. Why? |
| Answer» SOLUTION :The Plaster of Paris should be stored in a moisture-proof CONTAINER as it turns into a hard substance known as GYPSUM on absorption of WATER from moisture. | |
| 1122. |
Plasma. |
| Answer» SOLUTION :The state that CONSISTS of SUPER enegetic and super excited particles in the FORM of ionised gas is called PLASMA. Or The ionised gas is called plasma. | |
| 1123. |
Pinku took two closed vessels for carrying out two different reversible reactions. The first reaction was the thermal decomposition of cupric nitrate and the second reaction was the synthesis of iodine pentafluoride from its constituents. After the reactions reached the state of equilibrium, he changed the pressure on both the reaction mixtures keeping the other parameters unaltered. In the first reaction vessel, he observed that the intensity of brown colour had been decreasing and in the other reaction vessel, the intensity of purple colour had been increasing. Explain the reason behind such observation. |
|
Answer» Solution :Decomposition of cupric nitrate `2Cu(NO_(3))_(2)hArr 2CuO+4NO_(2(g))+O_(2(g))` Brwon Synthesis of iodine pentafluoride, `I_(2(g))+5F_(2(g)) hArr 2IF_(5(g))` Purple Since the intensity of brown colour was decreasing in the first reaction, it can be concluded that the PRESSURE on the equilibrium mixture increased. Since the intensity of purple colour was increasing in the second reaction, it can concluded that the pressure on the equilibrium mixture decreased. According to LE Chatelier's principle, if the pressure increases, the reaction proceeds in a DIRECTION in which the number of moles decreases and vice versa. |
|
| 1124. |
Pig iron obtained from blast furnace cannot be used for making tools because. |
|
Answer» HIGH percentage of IMPURITIES decrease malleability |
|
| 1125. |
Phosphorous reacts with air and forms P_2O_5. |
|
Answer» |
|
| 1126. |
Phosphor bronze is an alloy of copper with 3.5 percent to 10 per cent of tin and up to 1 per cent of phosphorous . What is the reason for the addition of phosphorous |
|
Answer» Solution :(i) bonds formed in the alloy (ii) reactivity of phosphorous with metals (iii) NATURE of prodcuts formed (iv) effect of these products on the physical PROPERTIES of the alloy. |
|
| 1127. |
Petroleum franction of which of thr following compositions can be used as a lubricating oil ? |
|
Answer» `C_5-C_7` |
|
| 1128. |
Petrol used in automobiles obtained by fractional distillation is less preferred over the petrol obtained by c.racking. Justify. |
|
Answer» Solution :(i) comparison of the componentspersent in petrol obtained by fractional DISTILLATION and by cracking (ii) comparisonof products obtained on fractional distillation and cracking of petrol (iii) effect of the products on the characteristic reaction INVOLVED in the usage of petrol as FUEL (iv) comparison of type and nature of products produced by the two types of petrol (v) comparison of effect of these products obtained on the environment (VI) comparison of effect of products obtained on knocking property of engines |
|
| 1129. |
PCl_(5) can be formed by heating a mixture of Cl_(2) " and " PCl_(3). If 1 mole each of Cl_(2) " and " PCl_(3) are mixed and the reaction is allowed to take place in a closed container fitted with a pressure gauge and explain with a suitable reason. |
| Answer» SOLUTION :`PCl_(3(G))+Cl_(2(g)) hArr PCl_(5(g))`. The number of moles of products is half the number of moles of the reactants. If the reaction GOES to completion, we should expect that the pressure indicator should show half of the initial pressure. But the pressure indicator indicates a HIGHER pressure than half of the initial pressure and it is less than the initial pressure. This indicates that the reaction did not go to completion. Later if the pressure indicator shows constant value without further RISE or fall in pressure, it implies that the reaction is in equilibrium. The pressure gauge inititally shows decrease followed by an increase ultimately reaching a constant position which indicates the equilibrium. | |
| 1130. |
Pawan is a student of class IX. The students of his class have recently studied the topic on the separation of various types of mixtures. One day, when the students were performing experiements in the science laboratory, their chemistry teacher Mr. Jain came to the laboratory with a packet in this hand. Mr. Jain told Pawan that the packet contained a mixture of aluminium powder, sulphur powder and nuckel powder. He asked Pawan to separate all the three constituents of this mixtures. Mr. Jain allowed the use of any other equipment/device/chemical, etc., required for this purpose from the laboratory. Pawan thought over the methods to be used for separating the given mixture for a while and then started working on the separation of mixture. He succeeded all the constituents of the mixture, one by one. Mr. Jain appreciated his effort. (a) Which property of sulphur powder could be used by Pawan to separate it from aluminium powder and nickel powder ? (b) Describe briefly, how Pawan separated sulphur powder from the mixture of aluminium powder, sulphur powder and nickel powder (c) Which property of nickel powder could be used by Pawan to separate it from aluminium powder ? (d) Describe briefly, how Pawan separated nickel powder from aluminium powder. (e) What values are displayed by Pawan in this episode ? |
|
Answer» SOLUTION :(a) Sulphur is SOLUBLE in an organic liquid 'carbon disulphide' (where aluminium powder and nickel powder are not solution in carbon disulphide). So, the property of solubility of sulphur powder in carbon disulphide is used to separate it from aluminium powder and nickel powder (b) The MIXTURE of aluminium powder, sulphur powder and nickel powder is taken in a test-tube and shaken with some carbon disulphide. Sulphur powder present in the mixture dissolves in carbon disulphide. On filtering, aluminium powder and nickel powder remain behind on the filter paper as residue and sulphur dissolved in carbon disulphide is obtained as a filtrate. On evaporating the filtrate, carbon disulphide is eliminated and sulphur powder is left behind (C) Nickel is a magnetic material which is attracted by a magnet (whereas aluminium is a non-magnetic material which is not attracted by a magnet). So, the magnetic property of nickel is used to separate nickel powder from aluminium powder (d) The mixture containing aluminium powder is taken in a watch glass. A horse-shoe type magnet is moved in the mixture of aluminium powder and nickel powder repeatedly. The nickel powder is attracted by the magnet, it clings to the poles of the magnet and gets separated. Aluminium powder is not attracted by the magnet. So, aluminium powder remains behind (E) The various values displayed by Pawn in this episode are (i) Knowledge of the solubility of sulphur in carbon disulphide , magnetic nature of nickel and non-magnetic nature of aluminium, and (ii) Application of knowledge in solving real-life problems. |
|
| 1132. |
Oxygen is very essential for us to live, It forms 21% of air by volume. Is it an element of compound? |
| Answer» Solution :OXYGEN is an ELEMENT, It contains the atoms of oxygen of the same KIND. | |
| 1133. |
Oxidising agents are also called as .................because they remove electrons from other substances. (a) electron donors (b) electron acceptors. |
|
Answer» |
|
| 1134. |
Out of proton and electron which is resposibel for the characteristics of the elements. |
| Answer» | |
| 1135. |
Out of boiling and evaporation, which is a surface phenomenon ? Explain. |
| Answer» Solution :EVAPORATION is as surface whereas BOILING once started occurs THROUGHOUT the liquid. | |
| 1136. |
Osmosis is a special kind of diffusion'. Comment. |
| Answer» Solution :YES, the statement is true. In both the CASES, there is movement of particles. However, osmosis is restricted to only liquid solutions in which the solvent molecules can pass through the PORES of a semi-permeable membrane while solute particles are unable to do so. Diffusion is very common in gases and also TAKES place in liquids. But no semi-permeable membrane is needed in case of diffusion. | |
| 1137. |
One of the indications that atoms are not indivisible comes from the study of ........ . (static electrictty, scattering of alpha-particles, seeds in the watermelon) |
|
Answer» |
|
| 1138. |
One of the following does not undergo sublimation. This one is : |
|
Answer» iodine |
|
| 1139. |
One Nanometer is ………………. . |
|
Answer» `10^(-7)` METRE |
|
| 1140. |
One mole of PCl_(5) is subjected to heating in a 1 L vessel. The number of moles of PCl_(3) formed at equilibrium is 0.6. Calculate the equilibrium constant for the dissociation of PCl_(5). |
| Answer» | |
| 1141. |
One mole of asubstance respresents one gram-formula mass of the substance . |
|
Answer» |
|
| 1142. |
One day Vabhav was performing experiments in the science laboratory based on the of chemical combination. Just then his chemistry teacher, Mr. Rajeev, came into the laboratory. Mr. Rajeev told Vaibhav that in an experiment conducted byby a class IX student when 10.6 g of sodium carbonate was reacted with 12.0g of ethanoic acid in a closed flask, then 16.4 g of sodium ethanoate, 4.4 g of carbon dioxide and an unknown mass of substances Y were produced. Mr. Rajeev asked Vaibhav to make use of this information and answer the following questions : (a) Write a word equation for the reaction which takes place on reacting sodium carbonate and ethanoic acid (b) Which is the substance Y which is produced in this reaction ? (c) What mass of substance Y is produced in thisreaction ? (d) Which law of chemical combination has been made use of in calculating the mass of substance Y ? State this law (e) What values are displayed by Vaibhav in this eposide ? |
|
Answer» SOLUTION :(a) When sodium carbonate and ethanoic acid react together, then sodium ethanoate, carnon dioxide and water are produced. A word equation for this REACION can be written as : `"Sodium carbonate" + "Ethanoic acid" rarr "Sodium ethanoate" + "Carbon dioxide"+ "Water (Y)"` (b) The substance Y produced in this reaction is 'water' (c) We will now calculate the mass of substance Y or mass of water produced in the given reaction. In this reaction, sodium carbonate and ethanoic acid are reactants whereas sodium ethanoate, carbon dioxide and water are products Here Mass of reaction = Mass of sodium carbonate + Mass of ethanoic acid `= 10.6 g + 12.0 g` `= 22.6 g`.....(1) Now, suppose the mass of substance Y is x So, Mass of products = Mass of sodium ethanoate + Mass of carbon dioxide + Mass of water `= 16.4 g +4.4 g +xg` `= 20.8 g + xg` ....(2) Now, according to the law of conservation of mass : Mass of products = Mass of reactants So, `20.8 +x 22.6` And `x = 22.6 - 20.8` `= 1.8 g` Thus, the mass of substance Y or mass of water produced is 1.8 GRAMS (d) (i) The law of conservation of mass in chemical reactions has been made use of in calculating the mass of substance Y or water (ii) The law of conservation of mass states that in a chemical reaction, the total mass of products is equal to the total mass of reactants. There is no charge in mass during a chemical reaction (e) The values displayed by Vaibhav in this episode are (i) Knowledge of the law of conservation of mass in chemical reactions and (ii) Ability to use this knowledge in solving problems. |
|
| 1143. |
One atomic mass unit = ......... |
|
Answer» mass of one ATOM of hydrogen |
|
| 1144. |
On the basis of Thomson's model of an atom, explain how the atom is neutral as a whole. |
| Answer» Solution :As PER Thomson.s MODEL of the atom, an atom consists both negative and positive charges which are EQUAL in NUMBER and magnitude. So they balance each other as a RESULT of which atom as a whole is electrically neutral. | |
| 1145. |
On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole. |
| Answer» Solution :According to Thomas 's MODEL of an atom an atom may be REGARDED as a POSITIVELY CHARGED shpere in which protons are presetn .The negatively charged electrons are believed to be studded or exbedded in the spher. Since the negative chages due to electons and positive charges due to protons balance each other the atom as a whole is neutral | |
| 1146. |
On the basis of Rutherford's model of an atom, which subatomic particles is present in the nucleus of an atom ? |
| Answer» Solution :On the BASIS of Rutherford.s model of an atom, the POSITIVELY charged PROTONS are present in the nucleus of an atom. | |
| 1147. |
On the basis of Rutherford's model of an atom, which subatomic particle is present in the nucleus of an atom ? |
| Answer» Solution :On the BASIS of Rutherford.s MODEL of an ATOM, proton are PRESENT inthe NUCLEUS of an atom. | |
| 1148. |
On the basis of Rutherford’s model of an atom, which sub- atomic particle is present in the nucleus of an atom? |
| Answer» SOLUTION :The nucleus of ATOM is positively CHARGED according to RUTHERFORD's model of an atom .All the protons in an atom re therefore present in the nucleus | |
| 1149. |
On suffering from high fever, which will lower your body temperature more , ice or ice cold water ? |
| Answer» Solution :ICE will lower the body temperature more than ice COLD water because latent heat of fusion of ice is QUITE high `(335 kJ kg^(-1))`. Ice is therefore, expected to absorb more heat ENERGY from the body and will lower the body temperature more than ice cold water. | |
| 1150. |
On keeping naphthalene balls open in air, its volume………….(increases, decreases, remains same) |
|
Answer» |
|