Saved Bookmarks
| 1. |
Explain smelting process. |
|
Answer» Solution :Smelting (in a Blast Furnace): The charge consisting of roasted ore, coke and limestone in the ratio 8:4:1 is smelted in a blast furnace by introducing it through the cup and cone arrangement at the TOP. There are three important regions in the furnace. (a) The Lower Region (Combustion ZONE): The temperature is at `1500^(@)C`. In this region, coke burns with oxygen to form `CO_(2)`when the charge comes in contact with a hot blast of air. `C + O_(2) underset(Delta)overset(1500^(@)C)to CO_(2)` + Heat It is an exothermic REACTION since heat is liberated. (b) The Middle Region (Fusion Zone) The temperatureprevails at `100^(@)C`. In this region, `CO_(2)` `CO_(2) + C underset(Delta)(overset(1000^(@)C)to 2CO -` Heat Limesteon decomposes to calcium oxide and `CO_(2)` `CaCO_(3) underset(Delta)to CaO + CO_(2)` - Heat These two reactions are enodthermic due to absorption of heat. Calcium oxide combines CaO + `SiO_(2) to CaSiO_(3)` (c ) The Upper Region (Reduction Zone) - The temperature prevails at `400^(@)C`. In this region carbon MONOXIDE reduces ferric oxide to form a fairly pure spongy iron. `Fe_(2)O_(3) + 3CO overset(400^(@)C)/(to 2Fe + 3CO_(2)` The molten iron is collected at the bottom of hte furnance after removing the slag. The iron thus formed is called pig iron. It is remelted and cast into different moulds. This iron is called cast iron. 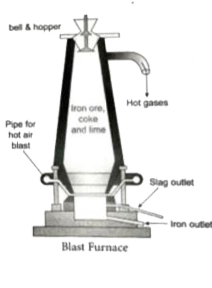
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?