Saved Bookmarks
| 1. |
Explain the nature of the covalent bond using the bond formation in CH_(3)Cl. |
Answer» Solution :`CH_(3)Cl` is chloromethane. It is made up of one carbon atom, three hydrogen atoms and one CHLORINE atom. Carbon atom has 4 outermost electrons, each hydrogen atom has 1 electron and chlorine has 7 outermost electrons. Carbon shares its FOUR outermost electrons with three hydrogen atoms and one chlorine atom to form `CH_(3)Cl` as FOLLOWS: 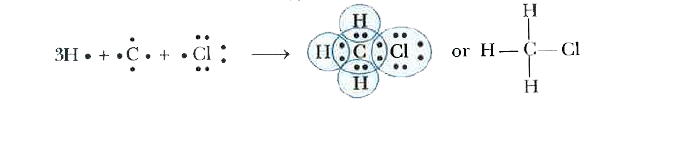 Each atom in `CH_(3)Cl` has a noble gas electron arrangement. Carbon attains the nearest noble gas CONFIGURATION of neaon, hydrogen acquires the configuration of HELIUM while chlorine achieves the configuration of argon. Chloromethane contains three C-H and one C-Cl covalent bonds. |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?