Saved Bookmarks
| 1. |
Find the gram molecular mass of the following from the data given : (i) H_(2)O (ii) CO_(2) (iii) NaOH (iv) NO_(2) (v) H_(2)SO_(4) |
Answer» Solution :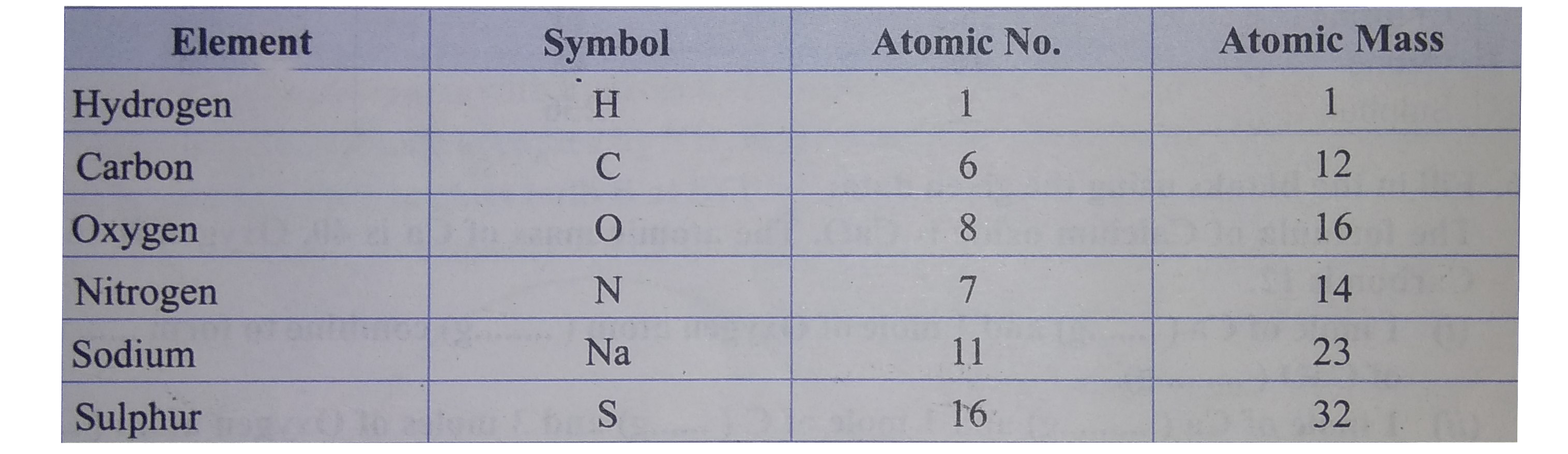 (i) `H_(2)O` Molecular MASS of `H_(2)O` `"Atomic mass of 2(H)"=2xx1=2` `"Atomic mass of 1(O)"=1xx16={:(16),(ulbar(18)):}` `therefore "Molecular mass of "H_(2)O=18g` (ii) `CO_(2)` `"Atomic mass of 1(C )"=1xx12=12` `"Atomic mass of 2(O)"=2xx16={:(32),(ulbar(32)):}` `therefore"Molecular mass of "CO_(2)=44g` (iii) `NaOH` `"Atomic mass of 1 (Na)"=1xx23=23` `"Atomic mass of 1(O)"=1xx16=16` `"Atomic mass of 1(H)"=1xx1={:(1),(ulbar(40)):}` `therefore"Molecular mass of NaOH"=40g` (IV) `NO_(2)` `"Atomic mass of 1(N)"=1xx14=14` `"Atomic mass of 2(O)"=2xx16={:(32),(ulbar(46)):}` `therefore"Molecular mass of "NO_(2)=46g` (v) `H_(2)SO_(4)` `"Atomic mass of 2(H)"=2xx1=2` `"Atomic mass of 1(S)"=1xx32=32` `"Atomic mass of 4(C )"=4xx16={:(64),(ulbar(98)):}` `therefore" Molecular mass of "H_(2)SO_(4)=98g` |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?