Saved Bookmarks
| 1. |
Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9, respectively. Which solution is : (a) neutral ? (b) strongly alkaline ? (c) strongly acidic ? (d) weakly acidic ? (e) weakly alkaline ? Arrange the pH in increasing order of hydrogen-ion concentration. |
|
Answer» SOLUTION :A solution having pH equal to 7 is neutral. A solution with pH RANGE 0 - 7 is acidic while a solution with pH range 7 – 14 is basic. Smaller the value of pH in the range 0 – 7, stronger is the acid. Higher the value of pH in range 7 - 14, stronger is the base (alkali). Based on above, we can DECIDE the acidic or basic nature of A, B, C, D and E: 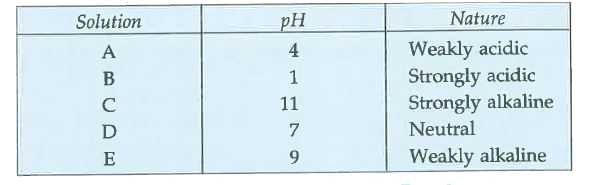 The pH in increasing ORDER of hydrogen ion concentration are : 
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?