Saved Bookmarks
| 1. |
Give reasons for the following:(i) Zinc oxide is considered an amphoteric oxide. (ii) Non-metals in general do not displace hydrogen from dilute acids. (iii) Metals conduct electricity. |
Answer» SOLUTION :(i) Zinc oxide reacts with both ACIDS and alkalis. Therefore, it is an AMPHOTERIC oxide.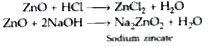 (ii) Non-METALS cannot provide ELECTRONS to convert H+ ions into hydrogen gas.(iii) Metals conduct electricity with the help of free or mobile electrons. |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?