Saved Bookmarks
| 1. |
The atomic number of tin is 50 and chlorine is 17. What should be the shape of SnCl_(2) molecule in its vapour state? |
Answer» Solution :The electronic configuration of Sn is `1s^(2)2S^(2)2p-.^(6)3s^(2)3p^(6)3d^(10)4s^(2)4p^(6)4d^(10)5s^(2)5p^(2)`and chlorine is `1s^(2)2s^(2)2p^(6)3s^(2)3p^(5)`.Hence, in `SnCl_(2)` molecule in vapour state, there is ONE lone pair of electrons and two BOND pairs of electrons in the valence shell of tin. Therefore, the expected shape is trigonal planar. But due to presence of the lone pair, its shape is bent and bond angle is `88^(@)`.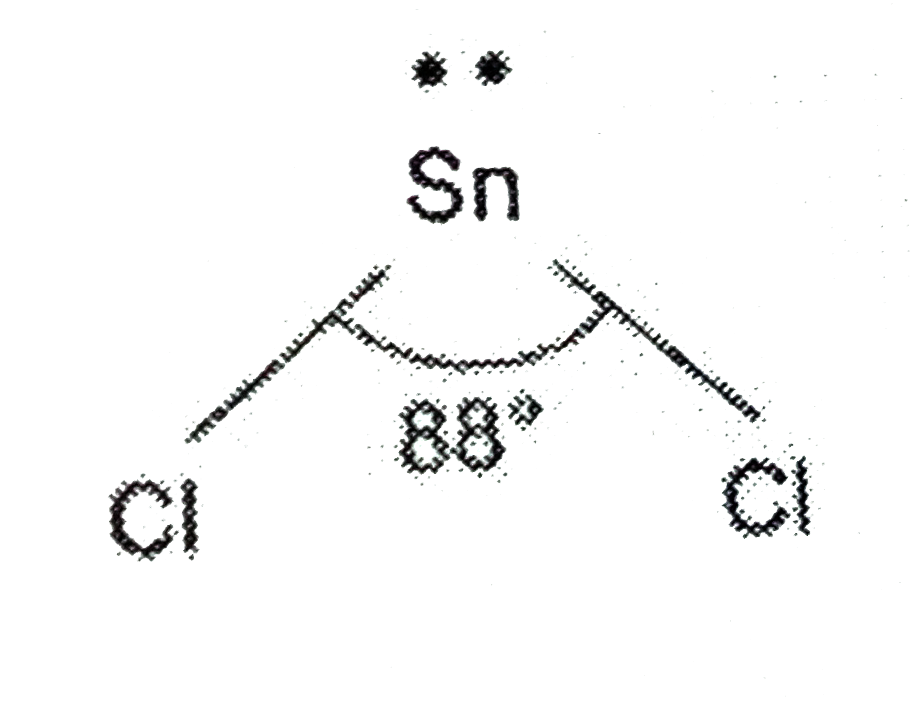
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the name given to a pure substance with only one kind of atoms?
- What is the difference between pure gold and 22 carat gold? Which type of gold is used for making ornaments ?
- The term pH was coined by ______
- Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- Which of the following metal is not found in a free state ?
- Why are alcohols poor conductors of electricity ?
- Which one of the following element is used as the standard for measuring the relativeatomic mass of an element in now a days?
- The thickness of _______ increases in the electrorefming of copper
- What do you think would happen when a mixture of iron filings and sulphur powder is heated ?
- What is the formula of the next homologue of propene (C_3H_6)?