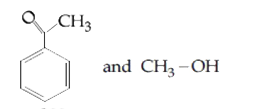Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 25151. |
The proper value of significant figures in 38.0+0.0035 +0.00003 is: |
|
Answer» 38 |
|
| 25152. |
The proper rays for radiocarbon dating are |
|
Answer» UV-rays |
|
| 25153. |
The propellant in the proposed PSL V rocket of the Indian space programme shall consist of |
|
Answer» A composite solid propellant |
|
| 25154. |
The projectile, that experiences minimum repulsion on approaching a particular nucleus is |
| Answer» Answer :A | |
| 25155. |
The progressive warming up of the earth surface is mainly due to : |
|
Answer» AUTOMOBILE exhaust |
|
| 25156. |
The progressive increase in the boilingpoints from CH_(3)Cl(249 K), CH_(3)Br (277 K) and CH_(3)I (316 K) is due to |
|
Answer» higher polarity of C - I bond and higher molar MASS |
|
| 25157. |
The progress of the reaction given below, CH_(3)COOC_(2)H_(5)+H_(2)Ooverset(H^(+))(to)CH_(3)COOH+C_(2)H_(5)OH can be followed by measuring the concentration of acid (HCl acid used as catalyst plus acetic acid formed during the reaction) by means of alkali titration. Find the volume of alkali (NaOH) needed for the end point that will increase with time. |
|
Answer» Solution :`{:(,CH_(3)COOC_(2)H_(5),+,H_(2)O,overset(H^(+))(to),CH_(3)COOH,+,C_(2)H_(5)OH),("At t=0",a,,"excess",,0,,0),("At t=t",a-x,,"constant",,x,,x),("At t=oo",0,,,,a,,a):}` If `V_(0)`, `V_(t)` and `V_(oo)` are the volumes of `NaOH` solution needed for the end point of titration of the reaction mixture at zero time, time `t` and at infinity, i.e. after completion of the reaction-the CONDITION being achieved by HEATING the reaction mixture for some time, then `V_(0) prop ["acid catalyst"]` `V_(t) prop ["acid catalyst"]+x` `V_(oo) prop ["acid catalyst"]+a` `:.V_(00)-V_(t) prop a-x` `V_(prop)-V_(0) prop a` (since concentration of `HCl` acid acting as catalyst will remain constant). The above reaction which is of first order (actually pseudo unimolecular) will, therefore obey following equation. `k=(2.303)/(t)log.(V_(oo)-V_(0))/(V_(oo)-V_(t))` |
|
| 25158. |
The progress of the reaction AiffnB , with time is presented in the figure. Determine (i) the value of n (ii) the equilibrium constant K (iii) the initial rate of conversion of A |
Answer» SOLUTION : At equilibrium Rate (forward) = Rate (backward) ` = (0.6 - 0.3)/(5)= (0.6)/(5) XX 1/n` For the eqb: `A IFF 2B, K =(0.6^2)/(0.3)` Initial rate = change in concentration of A in the 1ST hour 2,1.2,0.1 |
|
| 25159. |
The products X and Y in the following reaction are R-CH_(2)NO_(2) underset((ii) R'Br)overset((i) OH)(rarr) X overset(TiCl_(3))(rarr) Y respectively, |
|
Answer» `R R'CHNO_(2), R R'CO` 
|
|
| 25160. |
The products X and Z in the following sequence are |
|
Answer» iso - porpyl benzene and ACETONE 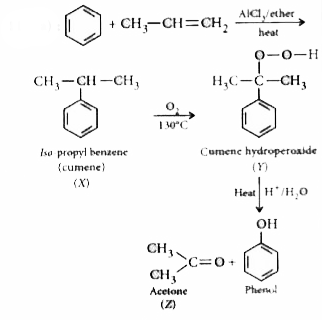
|
|
| 25161. |
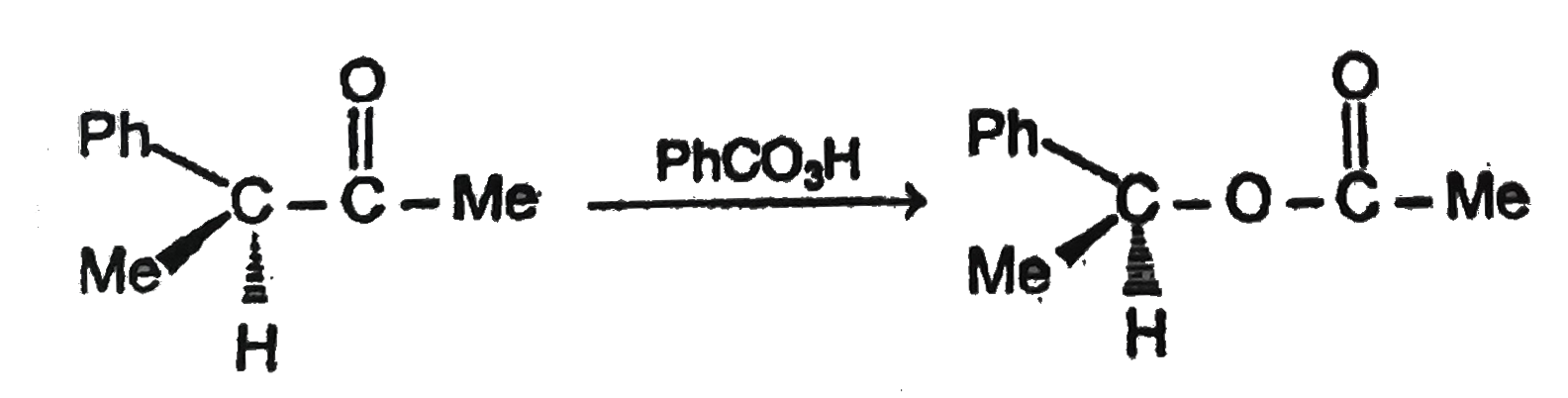
The products/s of the following reation is |
|
Answer»
|
|
| 25162. |
The products P and Q are |
|
Answer»
|
|
| 25163. |
The products ofreaelion of alcoholic silver nitrite witht ethyl bromide are |
|
Answer» ethane |
|
| 25164. |
The products of the reaction of two molecules of HCHO with struong potash is |
|
Answer» `CH_(3)OH " and "HCOOH` |
|
| 25165. |
The products of the reaction Al_4 C_3 + D_2O to …….+…… are : |
|
Answer» `C_2D_2 + AL(OD)_3` |
|
| 25166. |
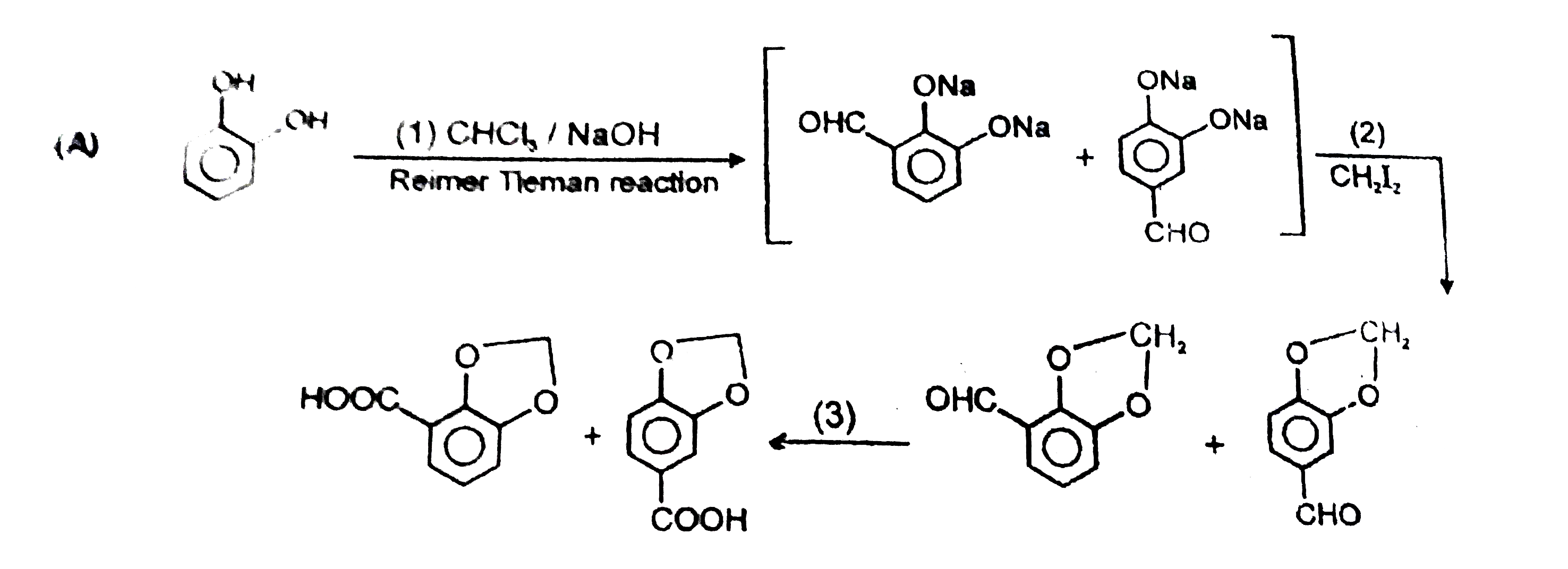
The products of the following reactions can be : |
|
Answer»
|
|
| 25167. |
The products of the following reaction are SiO_(2)+C overset(Delta)to |
|
Answer» SIC and `CO_(2)` |
|
| 25168. |
The products of the electrolysis of aq. Sodium chloride are |
|
Answer» SODIUM , CHLORIDE |
|
| 25169. |
The products of the electrolysis of concentrated aqueous solution of common salt are: |
|
Answer» `NA + Cl_2` |
|
| 25170. |
The products of reaction of alcoholic silver nitrite with ethyl bromide are: |
|
Answer» Ethane |
|
| 25171. |
The products of reaction of alcoholi silver nitrite with ethyl bromide are |
|
Answer» Ethane 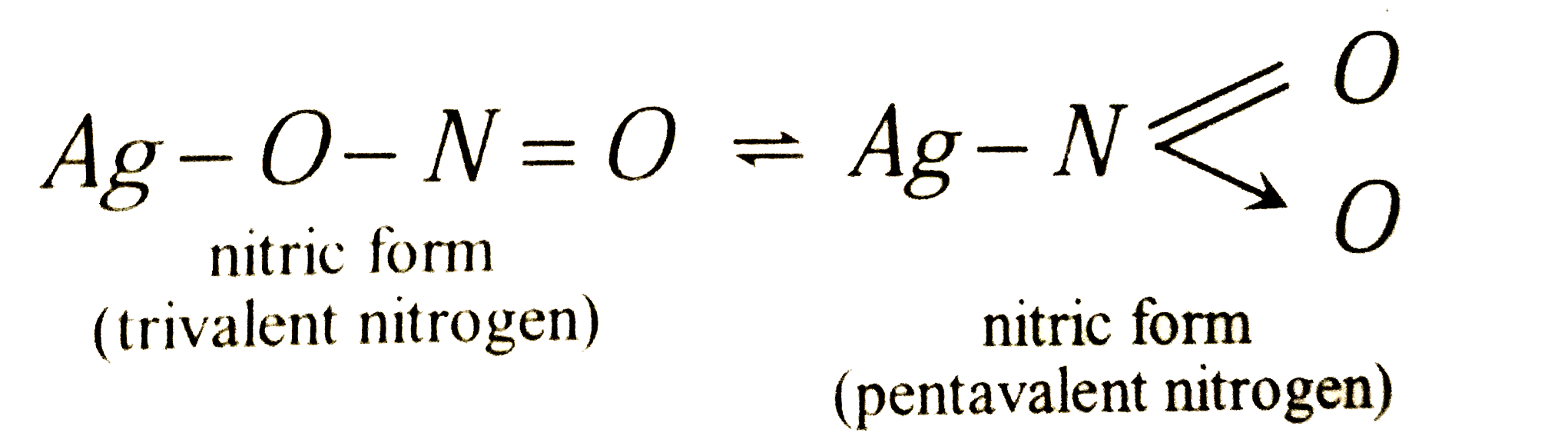 In other words, `NO_(2)^(-)` ions from `AgNO_(2)^(-)` may exist in two tautomeric FROMS `-O-N = O`(Nitrite ion) forming alkyl nitrities and  (nitro GROUP) forming nitroalkanes. (nitro GROUP) forming nitroalkanes. `2C_(2)H_(5)Br+2AgNO_(2) rarrC_(2)H_(5)NO_(2) + C_(2)H_(5)ONO + 2AgBr` |
|
| 25172. |
The products of reactio of alcoholic silver nitrite with ethyl bromide are |
|
Answer» Ethane 
|
|
| 25173. |
The products of oxidation of phosphorus acid by hot concentrated sulphuric acid are ...........and ............. . |
| Answer» SOLUTION :`H_(3)PO_(4), SO_(2) and H_(2)O`, i.e., `H_(3)PO_(3) + H_(2)SO_(4) overset(Delta)rarr H_(3)PO_(4) +SO_(2)+H_(2)O` | |
| 25174. |
The products of combustion of an aliphatic thiol (RSH) at 298 K are : |
|
Answer» `CO_(2)(g),H_(2)O(g)" and"So_(4)(g)` |
|
| 25175. |
The products of acid hydrolysis of P and Q can be distinguished by |
|
Answer» a. LUCAS reagent 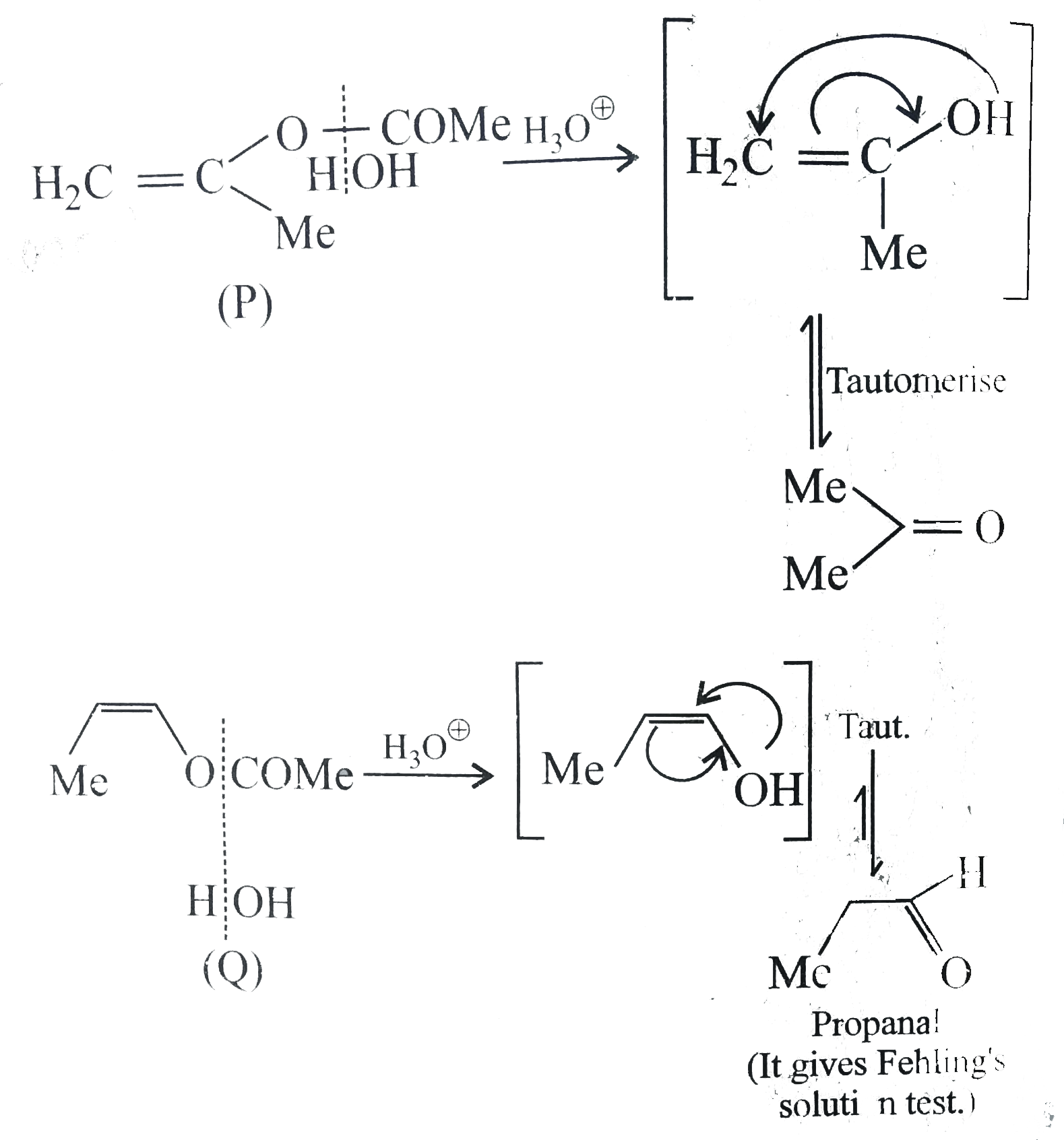
|
|
| 25176. |
The products of acid hydrolysis of P and Q can be distinguished by |
|
Answer» LUCAS reagent |
|
| 25177. |
The products of combustion of an aliphatic thil (RSH)at 298K are: |
|
Answer» `CO_2(L),H_2O(G) and SO_2(g)` |
|
| 25178. |
The products obtained when chlorine gas reacts with cold and dilute aqueous NaOH are |
|
Answer» `Cl^(-) and CLO^(-)` `Cl_(2) + 2 OH^(-) rarr Cl^(-) + OCl^(-) + H_(2)O` |
|
| 25179. |
The products obtained when benzyl phenylether is heated with HI in the mole rate 1:1 are. (1) Phenol. (2) benzyl alcohol, (3) benzyl,iodide, (4)Iodobenzene |
|
Answer» 1AND 3only |
|
| 25180. |
The products obtained when chlorine gas reacts with cold and dilute aqueous NaOH are ......... |
|
Answer» `CLO^(-)` and `ClO_(3)^(-)` |
|
| 25181. |
The products obtained when chlorine gas react with old and dilute aqueous NaOH are |
|
Answer» `ClO_(2)^(-)` and `ClO_(3)^(-)` Disproportional REACATION |
|
| 25182. |
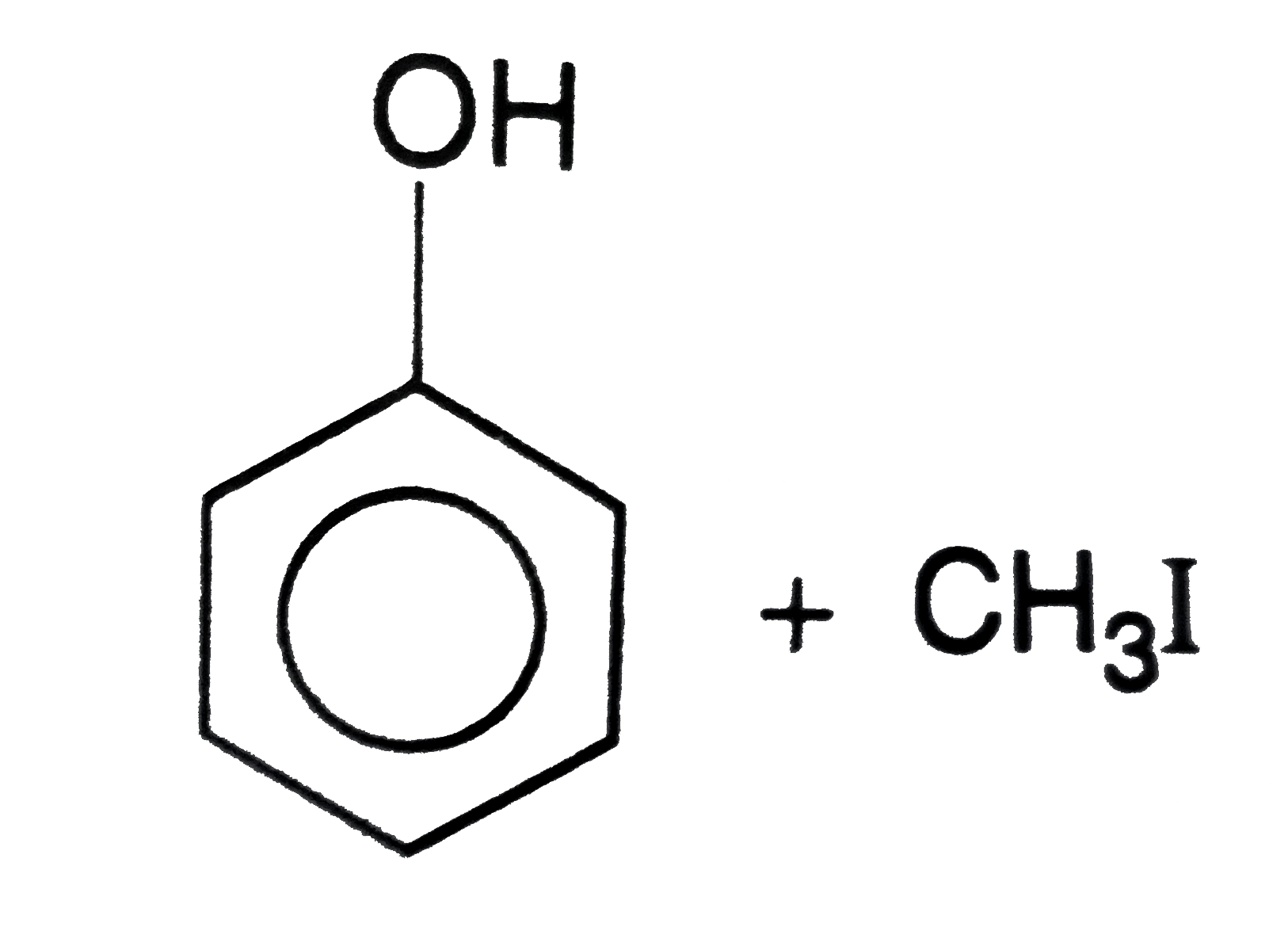
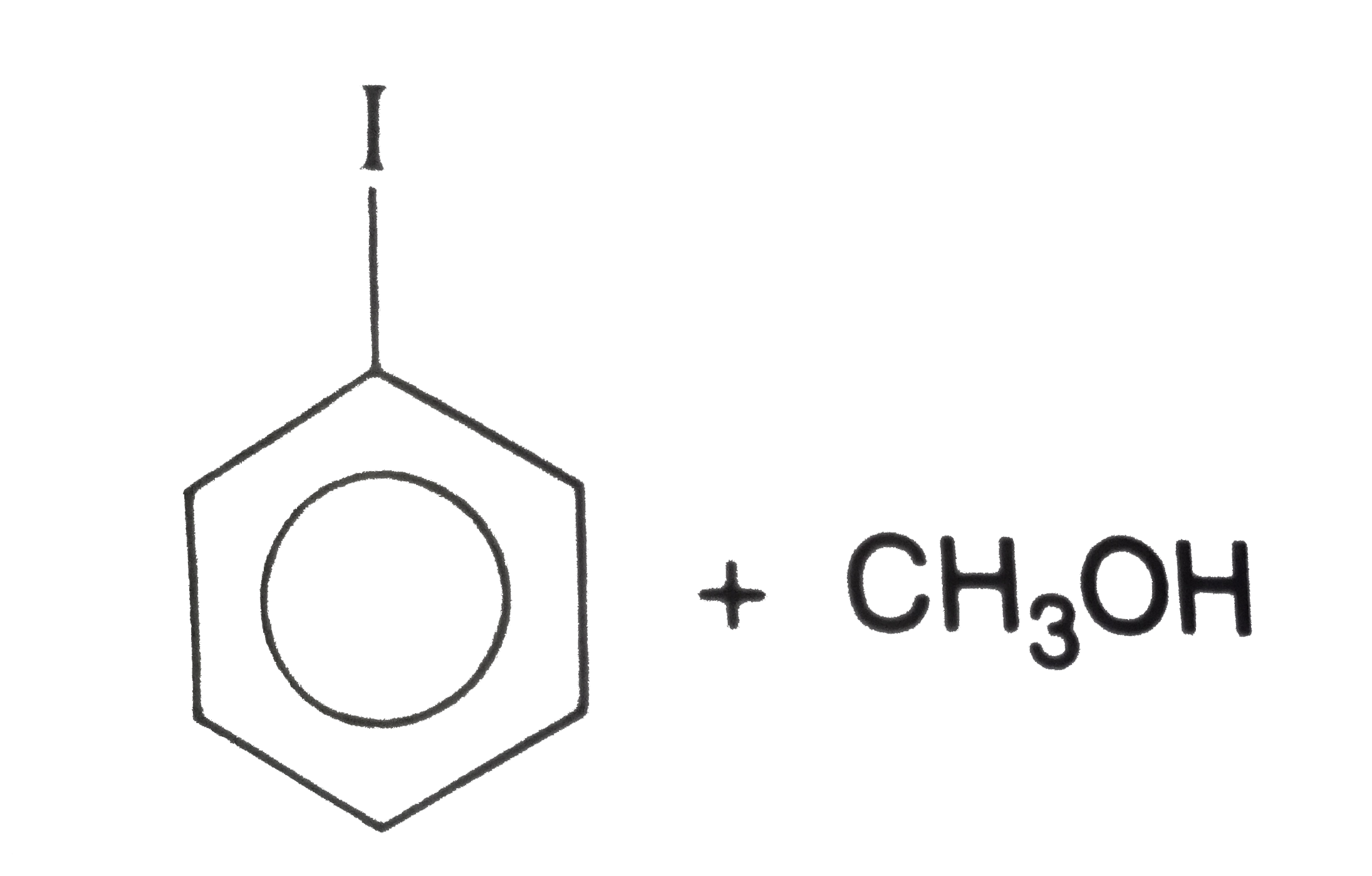
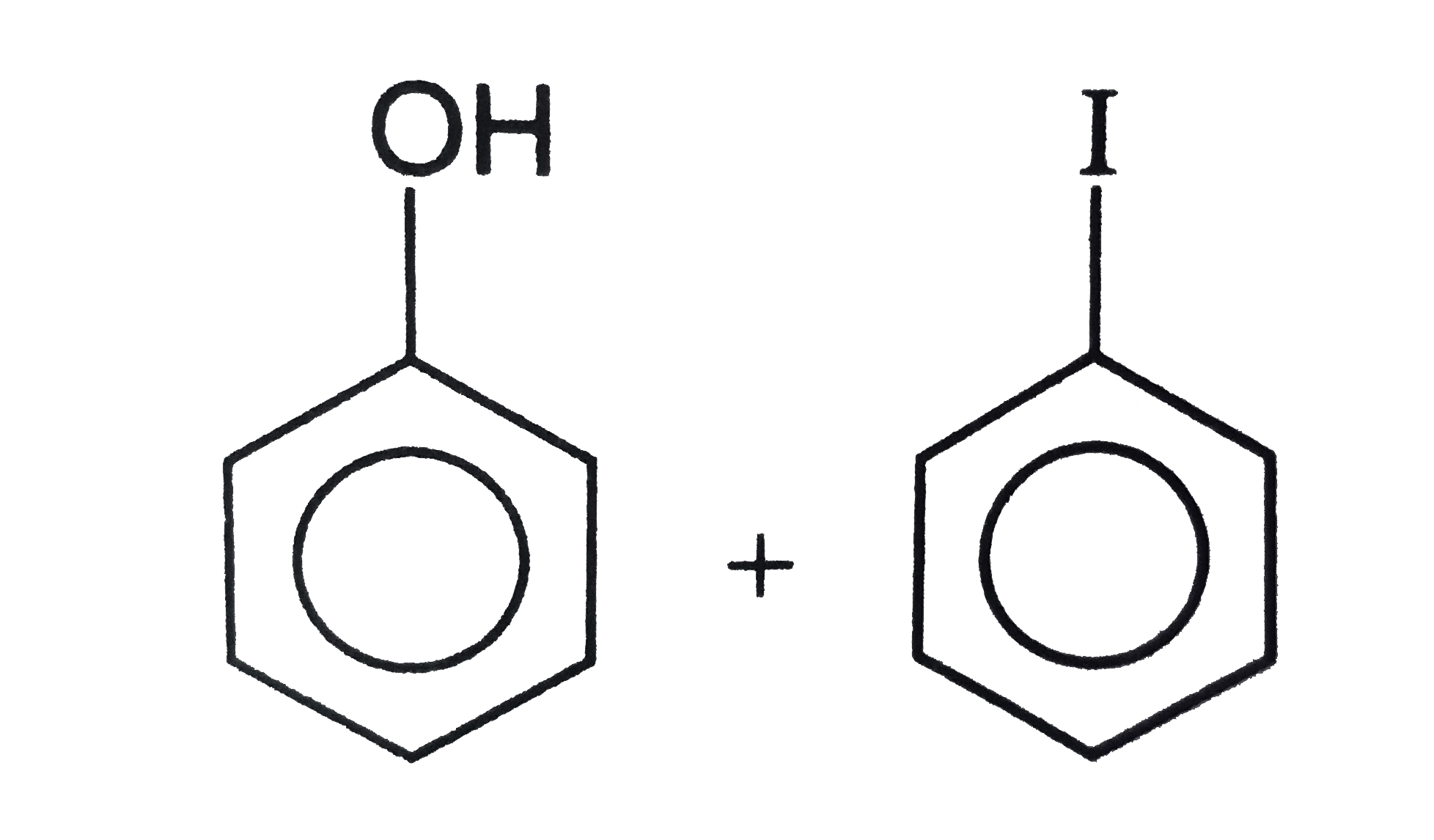
The products obtained when anisole is heated in a sealed tube with HI are |
|
Answer»

|
|
| 25183. |
The product(s) obtained via oxymercuration (HgSO_(4) + H_(2)SO_(4)) of 1-butyne would be: |
|
Answer» `CH_3-CH_2-oversetoverset(O)(||)C-CH_3` |
|
| 25184. |
The products obtained via oxymer curation (HgSO_(4)+H_(2)SO_(4)) of butyne would be |
|
Answer» `CH_(3)-CH_(2)-CO-CH_(3)` `CH_(3)CH_(2)C-=CH overset(HgSO_(4)+H_(2)SO_(4))to [CH_(3)CH_(2)overset(OH)overset(|)C=CH_(2)]toCH_(3)CH_(2)COCH_(3)` |
|
| 25185. |
The products obtained on the ozonolysis of pent-2-ene are : |
|
Answer» PROPANAL and ETHANAL 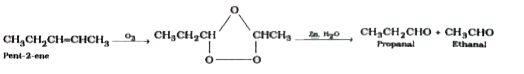
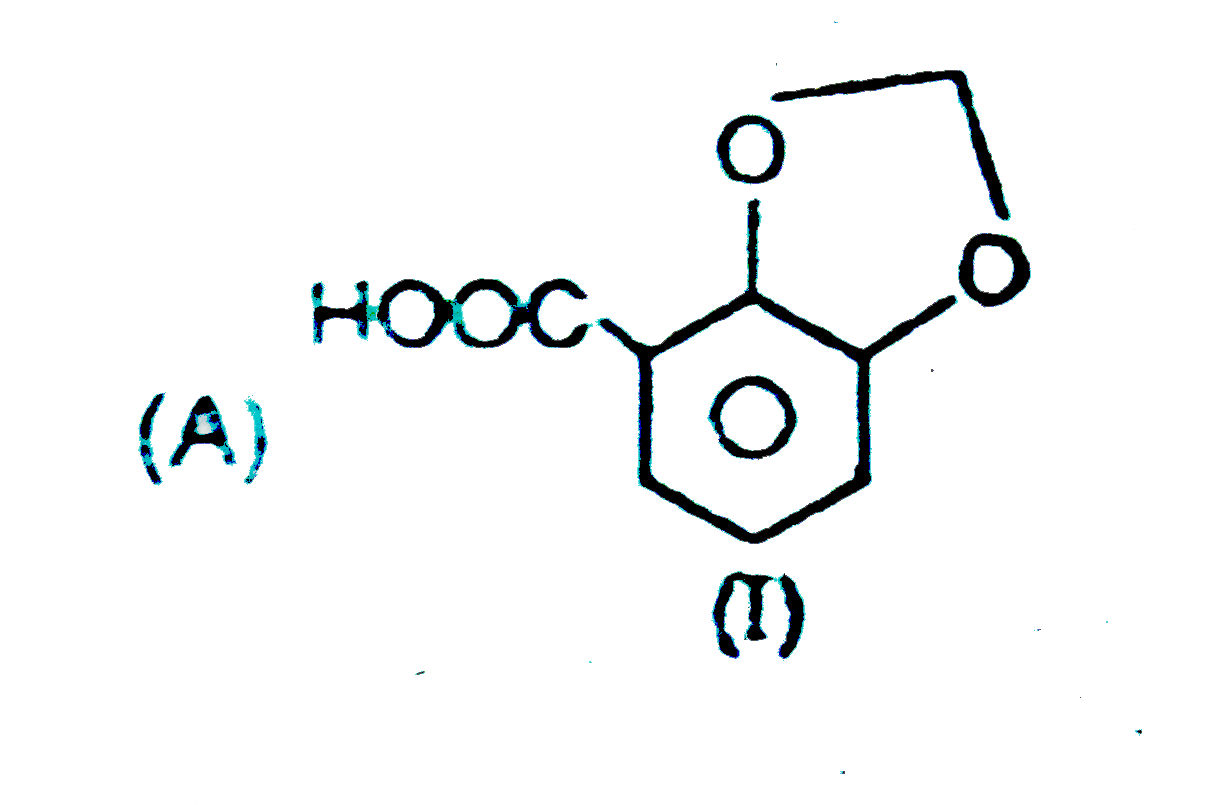
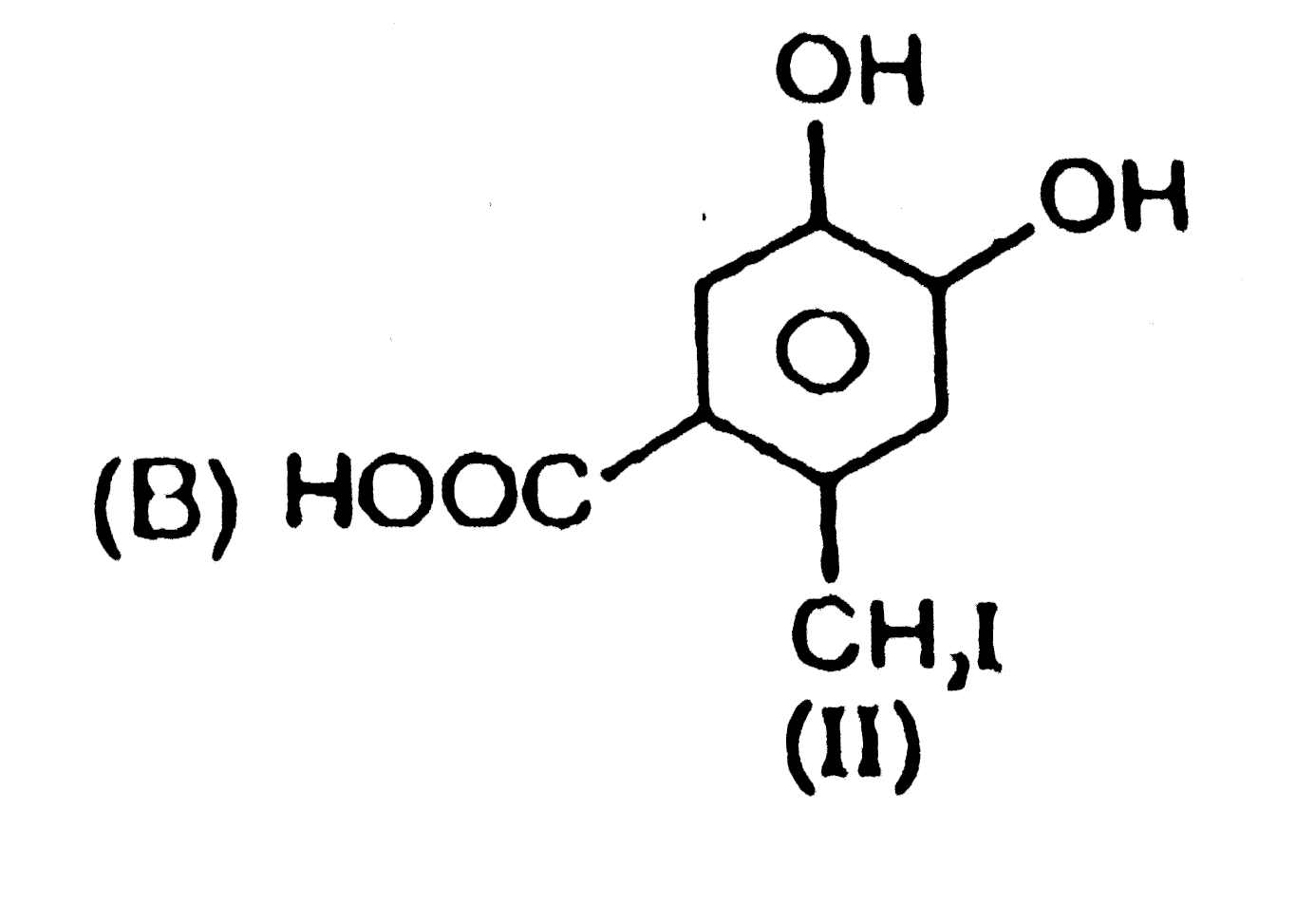
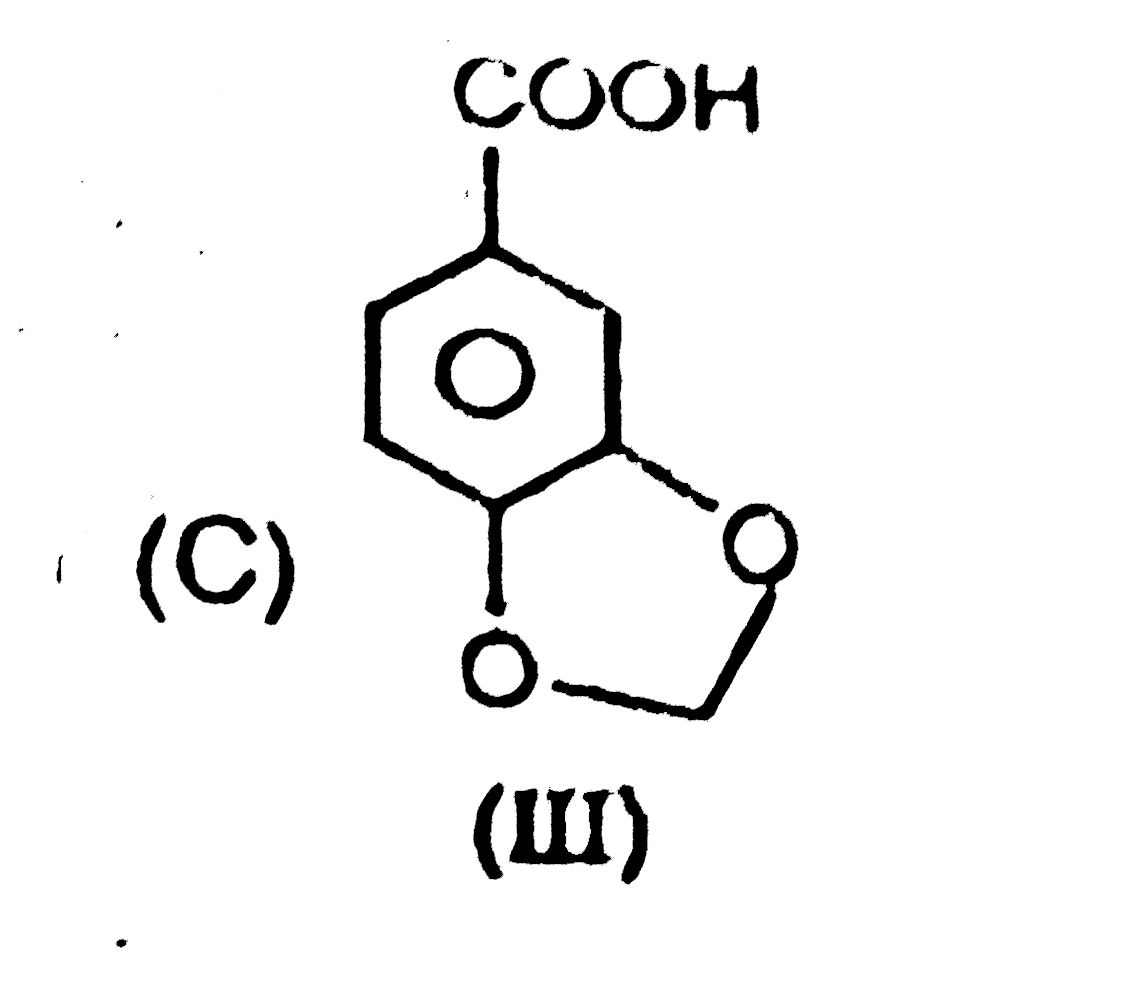
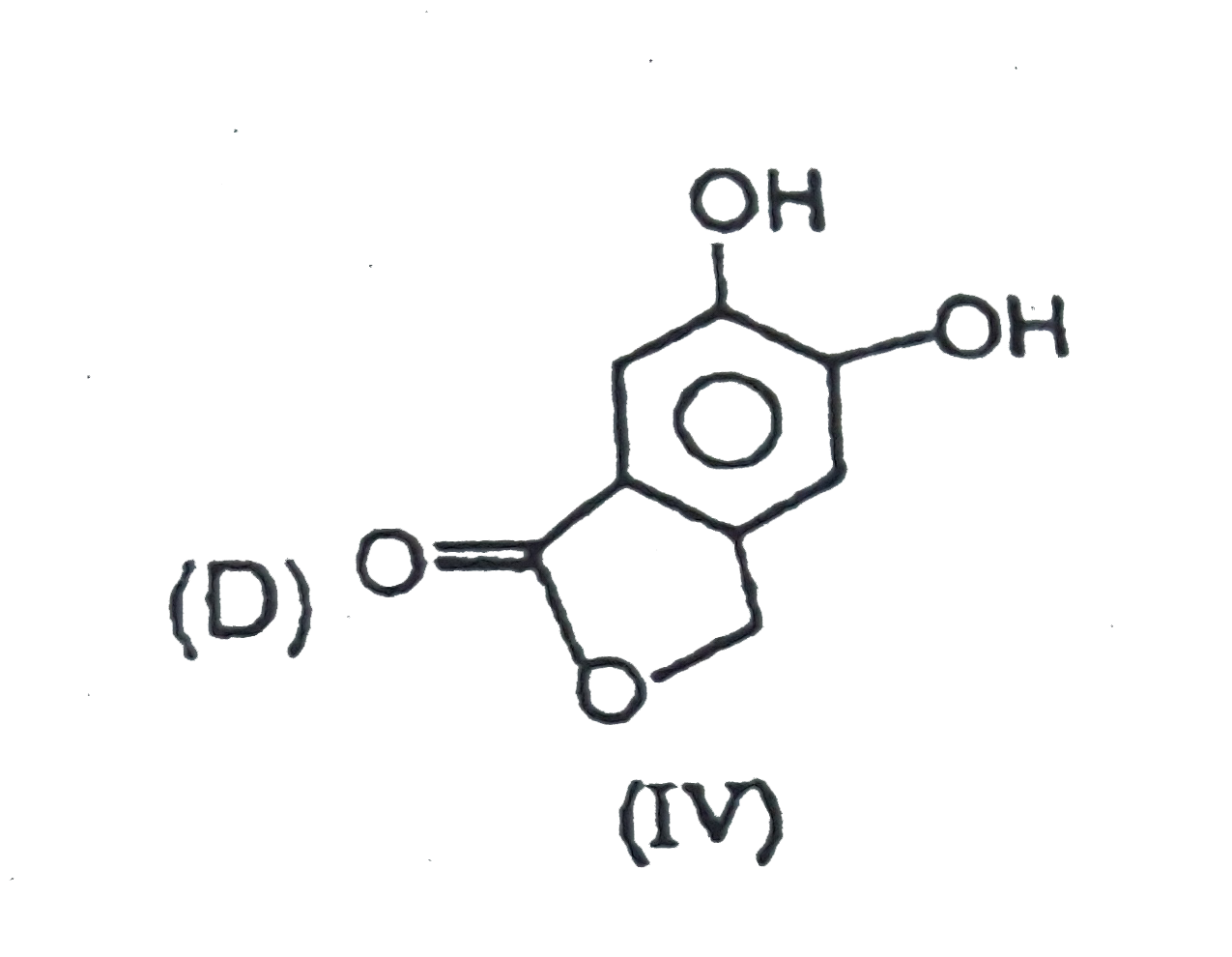
|
|
| 25186. |
The product obtained on reaction of C_2H_5Cl with hydrogen over palladium carbon is |
|
Answer» `C_3H_8` |
|
| 25187. |
The productsobtainedin thereacton , C_(6)H_(5)CH_(2) -CH_(3) underset(273K)overset(Cl_(2)liv)to is / are : |
|
Answer» `C_(6)H_(5)CHCl-CH_(3)` |
|
| 25188. |
The products obtained in the following reaction are underset(COOH)overset(CHO)(2|) underset(Delta)overset(NaOH)to |
|
Answer» `overset(CH_(2)OH)underset(COONa)(|)` `2underset(COOH)overset(CHO)(|) underset(Delta)overset(NaOH)to underset(COONa)overset(COONa)(|)+underset(COONa)overset(CH_(2)OH)(|)` |
|
| 25189. |
The productsformedwhen themixtureof methane and steamis passedoverfinelydividednickelat 1273K, are : |
|
Answer» `CO_(2) andH_(2) ` |
|
| 25190. |
The products obtained by means of HgSO_4 + H_2So_4 from 1-butyne would be : |
|
Answer» `CH_2CH_2CH_2CHO` |
|
| 25191. |
The products formed when propene is ozonolysed are ...... |
|
Answer» `HCHO + CH_3CHO` |
|
| 25192. |
The products formed when CH_3I,CH_3CHO and CH_3CH_2COOH are respectively reduced by HI in presence of red phosphorus are- |
|
Answer» `CH_3CH_3,CH_3CH_3,CH_3CH_2CH_3` |
|
| 25193. |
The products formed when Cl_2 reacts with excess of NH_3 are |
|
Answer» `NCl_(3)+HCL` |
|
| 25194. |
The products formed when bromocyclohexane and sodium propynide are heated together are |
|
Answer» 1-cyclohexylpropyne |
|
| 25195. |
The products formed on the cathode and the anode respectively on the electrolysis of an aqueous solution of MgSO_(4) between inert electrodes will be: |
|
Answer» `H_(2) (G) and O_(2) (g)` |
|
| 25197. |
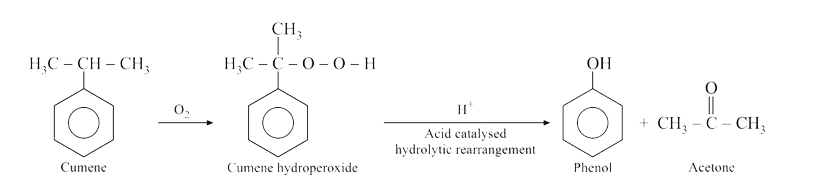
The products formed in the reaction of cumene with O_2followed by reactant with dil. HCl are : |
|
Answer»
|
|
| 25198. |
The products formed in the reaction are (AAK_MCP_36_NEET_CHE_E36_006_Q01) |
|
Answer»
|
|
| 25199. |
Complete the following equations: CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-O-CH_(3)+HI to |
|
Answer» `CH_(3)OH+ CH_(3)- UNDERSET(CH_(3))underset(|)overset(CH_(3))overset(|)C-I` `underset(underset("ether")("tert-Butymethyl"))(CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)C-OCH_(3))+HI overset(373 K)RARR underset(underset("alcohol")("Methyl"))(CH_(3)OH)+underset("tert-Butyl iodine")(CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)C-I)` |
|
| 25200. |
The products expected to be formed in the Wurtz reaction of a mixture of neopentyyl bromide and isobutyl bromide are (i) 2,2,4-trimethylpentane (ii) 2,2,5,5-tetramethylhexane (iii) 2,2,4,4-tetramethylhexane (iv) 2,5-dimethylhexane (v) 2,2,5-trimethylhexane |
|
Answer» (ii), (iii) and (v) `underset("2,2,5-Trimethylhexane (iv) (Cross product of A and B)")(+CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(.^(2)C)-CH_(2)-CH_(2)-overset(CH_(3))overset(|)(.^(5)C)H-CH_(3))` thus, option (b) is correct. |
|