Explore topic-wise InterviewSolutions in Current Affairs.
This section includes 7 InterviewSolutions, each offering curated multiple-choice questions to sharpen your Current Affairs knowledge and support exam preparation. Choose a topic below to get started.
| 1. |
Dust particles along with water vapour present in the atmophere help in the formation of clouds. |
|
Answer» |
|
| 2. |
During the summer vacations, Aryan went for a trip to Tirupathi in an old car along with his family. During the journey, the driver stopped the car beside a hotel and brought a can full of water and poured it into the radiator of the car. Aryan's son, Mouryan wanted to know the reason for pouring water into the radiator and asked his father who is a mechanialengineer by profession the reason. Aryan explained the role of water in the radiator of the car engine. Can you predict the explanation given by Aryan? |
| Answer» Solution :Specific heat CAPACITY of water is very high. Hence water can TAKE way a hot of heat from the hot engine WITHOUT SIGNIFICANT increase in its temperature. | |
| 3. |
During the conversion of surface water to potable water, chlorination is an important step. Give reasons. |
| Answer» SOLUTION :During chlorination, CHLORINE is ADDED to WATER, which produces nascent oxygen. This nascent oxygen kills all harmfull germs and BACTERIA present in water. | |
| 4. |
During summer vacation Ravi decided to go to his grandparent's place at Delhi. Since it was summer, therefore, Ravi's mother advised him to carry only cotton clothes. Why did she say so? Give reason. |
| Answer» Solution :COTTON has the PROPERTY absorbing sweat. When this is EXPOSED to atmosphere sweat undergose EVAPORATION which causes COOLING and hence we feel by wearing cotton clothes in summer. | |
| 5. |
During freezing heat is _________ . |
|
Answer» Freezing is the PROCESS of conversion of liquid to SOLID. This can be CARRIED out by EXTRACTING HEAT from liquid. |
|
| 6. |
Duralumin is used for the manufacture of aircraft because duralumin is |
|
Answer» a badconductor of heat and ELECTRICITY |
|
| 7. |
Duralumin contains ________ % of aluminium. |
|
Answer» DURALUMIN CONTAINS` 95%` of ALUMINIUM. |
|
| 8. |
Drinking water mainly contains small amounts of salts of ______, ______ and ______ metals. |
|
Answer» Drinking water mainly CONTAINS SMALL amounts of SALTS of sodium, magnesium and calcium METALS. |
|
| 10. |
Draw a neat line sketch of water cycle. |
Answer» SOLUTION :
|
|
| 11. |
Draw a neat labelled diagram for the electrolysis of water. |
Answer» SOLUTION :
|
|
| 12. |
Draw a line sketch of distribution of water on earth's surface. |
Answer» SOLUTION :
|
|
| 13. |
Dogs stretch out tongues generally in summer because |
|
Answer» evaporation LEAD cooling. |
|
| 14. |
Distinguishsoap from detergent. |
|
Answer» Solution :Soaps and detergent are two artificially SYNTHESIZED substances which areindispensable in our dialy activity. Soaps : Chemical composition wise soaps are thesodium salts of fatty acids. Examples of different fatty acids are olive oil, cotton seed oil, repeseed oil etc. When sodium hydroxide solution istreated with vegetables oil, soap isproduced. Soap forms PRECIPITATE in hard water and does not lather properly. Hence soap is INEFFECTIVE in hand water. Detergents : Detergents are sodiumsalt of alkyl hydrogen sulphate. Detergents do not form precipitates in hard water andcan be USED effectively in hand water. The cleansing of detergent is SUPERIOR to that of soap. Examples of a detergent is sodium lauryl sulphate. |
|
| 15. |
Distinguish sublimate from sublime. |
| Answer» SOLUTION :The process by which some SOLID substances directly into the vapour state without PASSING through the intermediate liquid state is called sublimation. The solid OBTAINED on cooling the vapour is called sublimate and the vapour FORMED is called sublime. | |
| 16. |
Distinguish between temporary hardness and permanent hardness. |
| Answer» Solution :`({:("Temperature hardness","""Permanent hardness"),("(1) It is caused by bicarbonate of calcium and magnesium","(1) It is caused by chlorides and sulphates of calcium and magnesium"),("(2) It can be REMOVED by BOILING, by the addition of washing soda and CLARK's method", "(2) It can be removed by the addition of washing soda, PERMUTIT PROCESS and ion exchange method."):})/` | |
| 17. |
Distinguish between malleability and ductility with an example. |
|
Answer» Solution :Malleability is the ability of substance to ROLL into sheets. GENERALLY, all METALS are malleable. Ductility is the ability of substance to drawn into thin WIRES. CARBON fibres are ductile in nature. |
|
| 18. |
Distinguish between melting and boiling. |
| Answer» Solution :The process which INVOLVES the change in state of matter from solid to liquid by heating is CALLED MELTING or fusion. The process which involves the conversion of liquid state to the GASEOUS state from the bulk of the liquid by heating is called boiling. | |
| 19. |
Distinguish between evaporation and boiling. |
Answer» SOLUTION :
|
|
| 20. |
Distinguish between compounds and mixtures. |
Answer» SOLUTION :
|
|
| 21. |
Distinguish between cations and anions. |
| Answer» Solution :`{:("Cations","Anions"),(1."Positively charged ions are CALLED cations.","Negatively charged ions are called anions"),(2."Generally metals FORM cations by LOSING ELECTRONS.","Generally nonmentals form anions by GAINING electrons."):}` | |
| 22. |
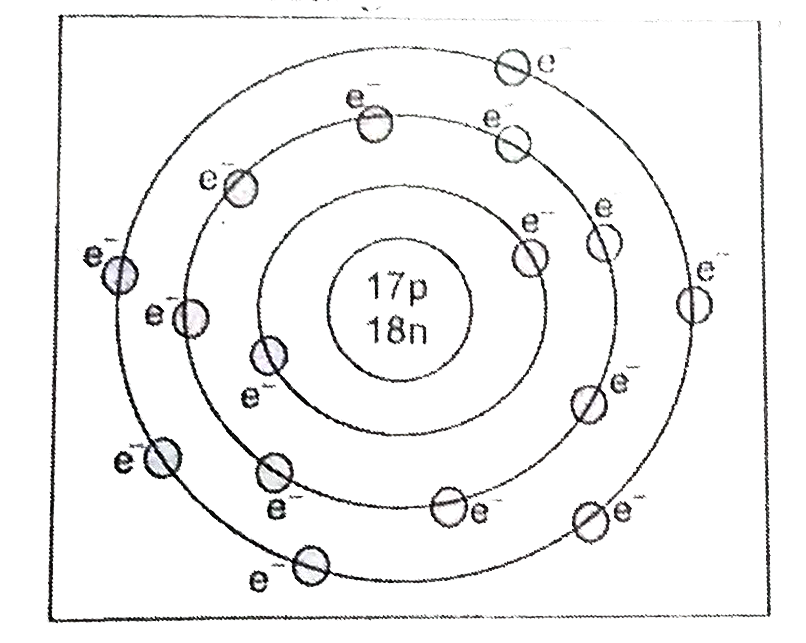
Distinguish between atomic number and mass number with an example. |
|
Answer» Solution :Atomic number and mass number : The number of protons present inside the nucleus is called atomic number . It is EQUAL to the number of ELECTRONS in a neutral atom . The ELEMENTS are characterized by their atomic numbers . Atomicnumber is denoted by Z. Example :Insodium , Z = 11 , MEANS that an atom of sodium contains 11 protons in the nucleus and 11 electrons outside the nucleus. Mass number : Mass number indicates the sum of protons and neutrons present inside the nucleus. it isdenoted by A. Example : In sodium, Z = 11 , A= 23 , indicates that an atom of sodium contains 11 proton and (23 -11 ) = 12 neutrons. |
|
| 23. |
Distinguish between atom and molecule. Give example of elements existing as atoms and molecules. |
|
Answer» Solution :Atom is the smallest particle of matter which may or may not exist independently. MOLECULE is the smallest particle of matter which can have INDEPENDENT EXISTENCE. Inert gases exist in ATOMIC STATE. Oxygen, nitrogen etc. exist in molecular from. |
|
| 24. |
Dissolution of calcium oxidein water is which type of reaction? |
| Answer» Solution :Dissolution of CALCIUM OXIDE in water is a COMPUND - compoundcombination reaction. | |
| 25. |
Discuss the various steps involved in making potable water. |
|
Answer» Solution :The purification of water basically involves two steps: (i) Removal of suspended impurities. (ii) Removal of microorganisms. (i) Removal of suspended impurities (a) Sedimentation: River water is PUMPED into settling tanks and ALLOWED to settle without any disturbance. The suspended impurities being larger particles settle down on standing. (b) Addition of chemicals: The above process makes water only semi clear. This is passed into MIXING chamber where alum and lime are added to water and it is agitated with huge mechanical blades. Later on, this water is pumped into sedimentation tank. Alum hastens the process of precipitation of suspended impurities. During the precipitation process, some bacteria also get trapped and removed. (c ) Filtration through sand and gravel: The clear water obtained is pumped into sand and gravel filter. This removes all remaining traces of suspended impurities. (ii) Removal of microorganisms Addition of bleaching powder releases chlorine which helps in killing the harmful bacteria and the pure water obtained is stored in storage tanks. The water so obtained at the end of the above purification process is devoid of suspended impurities and harmful bacteria. This water is fit for drinking and is called potable water. Potable water contains dissolved gases such as `O_(2)` and `CO_(2)` and also some dissolved salts especially common salt. These salts PLAY an IMPORTANTROLE in the metabolic activities. They also provide taste to water. Therefore this water is very much suitable for human consumption. 
|
|
| 26. |
Discuss the ganeral properties of glass. |
|
Answer» Solution :General PROPERTIES of glass: (i) Glass is a supercooled liquid. (ii)It is TRANSPARENT, HIGHLY resistant to chemicals and bad CONDUCTOR of heat and electricity. It can be easily moulded intodesired shapes. |
|
| 27. |
Discuss the characteristics of (a) element (b) mixture (c ) compound |
|
Answer» Solution :(a) Element : An element is a substance in a molecule which is constituted of only one kind of atoms and cannot be further divided by any physical or chemical means. Example : HYDROGEN, oxygen, chlorine, sodium, potassium etc. are the examples of elements. Molecules of these substances consist of only one type of atoms. Molecules of different elements may contain different number of atoms. (b) Mixture : A mixture is a substance which is formed by mixing two or more substances (elements, compounds or both) physically in any proportion. No chemical reaction takes place during the formation of a mixture and all the constituents of mixture retain their properties. Constituents of mixture can easily be separated by physical means. Example : solution of sugar and WATER is a mixture in which sugar and water both retain their properties and can be separated by a SIMPLE physical process that is evaporation. Muddy water is ANOTHER example of mixture of mud and water which can easily be separated by filtration. (C ) Compound : A compound is a substance which is formed due to the chemical combination of two or more elements in a definite proportion by mass. The constituents of do not retain their properties and they can be separated only by chemical means. Example : Water, carbon dioxide, hydrochloric acid, sodium chlocium carbonate etc, are a few examples of compounds. A molecule of carbon dioxide is constituted of a carbon atom and two oxygen atoms. Two atoms of hydrogen and one atom of oxygen combine chemically to form a molecule of water. |
|
| 28. |
Discuss five important physical properties of water. |
|
Answer» Solution :Physical properties of water Water possesses certain significant physical properties which are responsible for making it a vital liquid. (i) Nature: It is a colourless, odourless and tasteless liquid. (ii) FREEZING point:Water freezes to ice at `0^(@)C` under NORMAL atmospheric pressure. (iii) Boiling point: Water gets CONVERTED to steam at `100^(@)C` under normal atmospheric pressure. (iv) Specific heat: The amount of heat energy required to raise the temperature of unit capacity of substance through `1^(@)C` is called the specific heat capacity of that substance. Specific heat capacity of pure water is 1 `"calorie"//g^(@)C`. Among all substances, water has the highest specific heat capacity. (V) Latent heat: When a substance changes from one state to the other, certain amount of heat is required to break the intermolecular forces of attraction. For instance, when ice of certain mass at `0^(@)C` is converted to water of same mass at `0^(@)C`, the heat supplied brings about conversion of state without change in temperature. This is stored as potential energy in the water molecules and is called latent heat of fusion and is equal to 80 cal/g Similarly, the same conversion of 1 g of water at `100^(@)C` to water vapour requires 540 cal heat and this is called latent heat of vapourisation. At any temperature between `0^(@)C` and `100^(@)C`, conversionof water to water vapour takes PLACE rather slowly. This process is called evaporation. The rate of evaporation increases with increase in temperature. |
|
| 29. |
Discuss few applications of graphite. |
|
Answer» Solution :Applications of graphite (i) Pencil leads are manufactured by mixing graphite with clay. (ii) Graphite mixed with fine clay is moulded andsintered into crucibles of desired size and shape which are USED tomelt small quantities of METALS. (iii) It is also used as an excellent lubricant in those parts of the machineries where HIGH heat is generated due to friction. (IV) Graphite is used in electrolytic cells as electrodes. (v) Graphite is used in nuclear reactor as a moderatorto slow down theneutrons produced due to the splitting of the atoms or thorium. |
|
| 30. |
Difine doubledecomposition reaction . Give an example. |
|
Answer» Solution :The chemical reaction in which the two reactant (compounds) exchange their respective radicals and two other new compounds are FORMED is CALLED DOUBLE DECOMPOSITION reaction. Example :`NaCl+AgNO_(3)toNaNO_(3)+AgCl` |
|
| 31. |
Dibasic weak mineral acid among the following is |
|
Answer» acetic ACID |
|
| 32. |
Describe various sources of water. |
|
Answer» Solution :Sources of Water (a) Surface water: It is the water present on the surface of earth. The water present in oceans, seas, rivers, springs, etc, comes under surface water. Oceans and Seas: These are the largest sources of water. Almost 97% of water available on earth is present in oceans and seas. This water contains huge amount of dissolved salts in addition to other impurities. Due to the presence of high PROPORTION of dissolved salts (especially 2.5%common salt) which makes the water salty and renders the water unfit for usage and consumption, this water is called saline water. River and lakes: Rain water from HIGHER altitudes flows down the hills and forms rivers, Apart from this rain water, water formed by melting of ice and snow flows into the rivers. Since water takes a long course and flows over land surface from higher altitudes, it contains various impurities and SOLUBLE salts. As the proportion of dissolved salts is less than in saline water, it is considered as fresh water and is available for consumption for various activities. Rain water: It is the main source of natural water. Since it DIRECTLY comes from atmosphere, it is the purest form of water. The initial showers of rain water contain dust particles and other microorganisms. The later part of rain water is devoid of any impurities. Therefore it is considered as the purest form of natural water. (b) Underground water:A part of the rain water reaches the water bodies such as rivers and lakes. A part of the rain water seeps into the soil and reaches the bottom layers. The rain water as well as some water from river water seeps through the soil and FILLS the empty spaces and cracks in the layers of the earth. This phenomenon is called infiltration and the water is called underground water. As water infiltrates through the open spaces in the soil, it first passes through the zone of aeration where the soil is unsaturated. At greater depths known as recharge area water fills in more spaces until the zone of saturation is reached. At this level, the ground water pressure is equal to the atmospheric pressure and this is known as water table. Below the water table, there is an underground layer of water bearing permeable rock or unconsolidated materials from which ground water can be usefully extracted using a water well. This is called aquifer. |
|
| 33. |
Describe the various stages of purification of drinking water |
|
Answer» Solution :(i) Removal of suspended impurities (a) Sedimentation: River water is pumped into settling tanks and allowed to settle without any disturbance. The suspended impurities being larger particles settle down on standing. (b) Addition of chemicals:The above process makes water only semi clear. This is passed into mixing chamber where alum and lime are added to water and it is agitated with huge mechanicalblades. LATER on, this water is pumped into sedimentation tank. Alum hastens the process of precipitation of suspended impurities. During the precipitation process, some bacteria also get TRAPPED and removed. (c ) Filtration through SAND and gravel: The clear water obtained is pumped into sand and gravel filter. This removes all remaining traces of suspended impurities. (ii) Removal of microorganisms Addition of bleaching powder releases chlorine which helps in killing the harmful bacteria and the pure water obtained is STORED in STORAGE tanks. |
|
| 34. |
Describe the process of separation of constituents of gum powder. |
|
Answer» SOLUTION :Separation of the components`(KHO_(3)("nitre")+"carbon+sulphur) of gunpowder"` Procedure : Carbon disulphide is added to beaker containing gunpowder sulphur GETS DISSOLVED in carbon disulphide. Theis mixture is then filtered with the help of a filter paper. The filtrate obtianed taken in flat vessel and exposed to air till carbon disulphide vapourises away leaving behind sulphur. The residue obtained containing carbon powder and nitre. It is taken in a beaker containing hot water. Nitre dissolves in hot water This mixture is then filtered with the help the of filter paper. Carbon powder is left on the filter paper as a residue which is collected after DRYING. The filtrate on EVAPORATION to dryness by heating gives solid nitre. Thus the constituents of gunpowder are separated. |
|
| 35. |
Describe the process of galvanization. How is it useful ? |
| Answer» SOLUTION :It is used in the process of GALVANIZING ironsheets. In thegalvanization process, the hot iron sheets are DIPPED in molten zinc and then PASSED through hot and heavy rollers. On cooling, a coating of zinc metal is formed over the iron sheets. The coating of zinc protects iron from rusting and hence is used for themanufacture of ironranks, rain water, drain PIPES etc. | |
| 36. |
Describe the process of separation of mixture of iron fillings, camphor and sand. |
|
Answer» Solution :Separation of the components of a mixture of iron fillings, sand and camphor Procedure : The mixture of iron filling, sand and camphor spread evenly in basin. A STRONG bar magnet is brought close to the mixture repeatedly. The iron fillings attracted by the magnet. The iron fillings cling to the magnet can be shaved off from the surface with help of piece of cardboard. The remaining constituents are COVERED with a INVERTED funnel whose outside surface is wrapped with moist filter paper and the open end of the steam of the funnel is plugged with a piece of paper of cotton to stop the PASSAGE of any vapours formed. Then the mixture is GENTLY heated. Camphor undergoes sublimation and get deposited on the cooler inside wall of the funnel and later on it is scrapped off. Sand is left behind in the basin. Thus all the constituents of the given mixture are scparated by magnetic separation followed by sublimation |
|
| 37. |
Describe the methods of preparation of acids and bases with the help of examples. |
|
Answer» Solution :Methods of preparation of acids and bases Acids From non-METALS: Non-metallic elements on heating with oxygen give corresponding oxides. Some oxides on dissolution in water give the corresponding acids and hence such non-metallic oxides are called acidic oxides. Eamples: `C + O_(2) RARR CO_(2)` `""CO_(2) + H_(2)O rarr H_(2)CO_(3)` `""S + O_(2) rarr SO_(2)` `""SO_(2) + H_(2)O rarr H_(2)SO_(3)` `""2SO_(2) + O_(2) rarr 2SO_(3)` `""SO_(3) + H_(2)O rarr H_(2)SO_(4)` The oxides which are insoluble in water are called neutral oxides. Examples: NO, CO Bases From metals: Metals on heation with oxygen give metal oxides which are basic in nature. These metal oxides on dissolution in water give corresponding alkalies (water soluble BASE). Examples: `4Na + O_(2) rarr 2Na_(2)O` `""Na_(2)O + H_(2)O rarr 2NaOH` `""2Mg + O_(2) rarr 2MgO`, `""MgO + H_(2)O rarr Mg(OH)_(2)` |
|
| 38. |
Describe the laboratory preparation of oxygen gas from hydrogen peroxide with a neat diagrem. |
Answer» SOLUTION : <br> `Principle: Hydrogen peroxide in the presence of a catalyst, that is manganese dioxide `(MnO_(2))` undergoes decomposition to give water and oxygen at room temperature. <br> `2H_(2)O_(2)overset(MnO_(2))(to)2H_(2)O+O_(2)` <br> Experiment: `MnO_(2)` is taken in a clean and dry conical flask. `H_(2)O_(2)` solution is added drop wise through the hiistle funnel which is fitted to conical flask with the help of a rubber stopper. <br> Observation: The oxygen gas evolved passes through a delivery tube into the beehive shelf placed in a trough of water. The oxygen gas evolves with effervescenve in the gas cylinder inverted over the beehive shelf. The gas evolved rekindles a glowing splinter. <br> Method of collection of oxygen gas: `O_(2)` gas evolved is collected by downward displacement of water. <br> Precautions: (a) heating of reaction mixture should be avoided. <br> (b) `MnO_(2)` used should be devoid of impurities.)
|
|
| 39. |
Describe the laboratory method of preparation of hydrogen gas. |
|
Answer» Solution :Laboratory method of preparation Principle: All the metals present above HYDROGEN in the reactivity series on reaction with dilute HCI or dilute `H_(2)SO_(4)` LIBERATE hydrogen gas. Zinc metal is preferred for this reaction. `Zn+2HCItoZnCI_(2)+H_(2)uarr` `Zn+H_(2)SO_(4)toZnSO_(4)+H_(4)uarr` 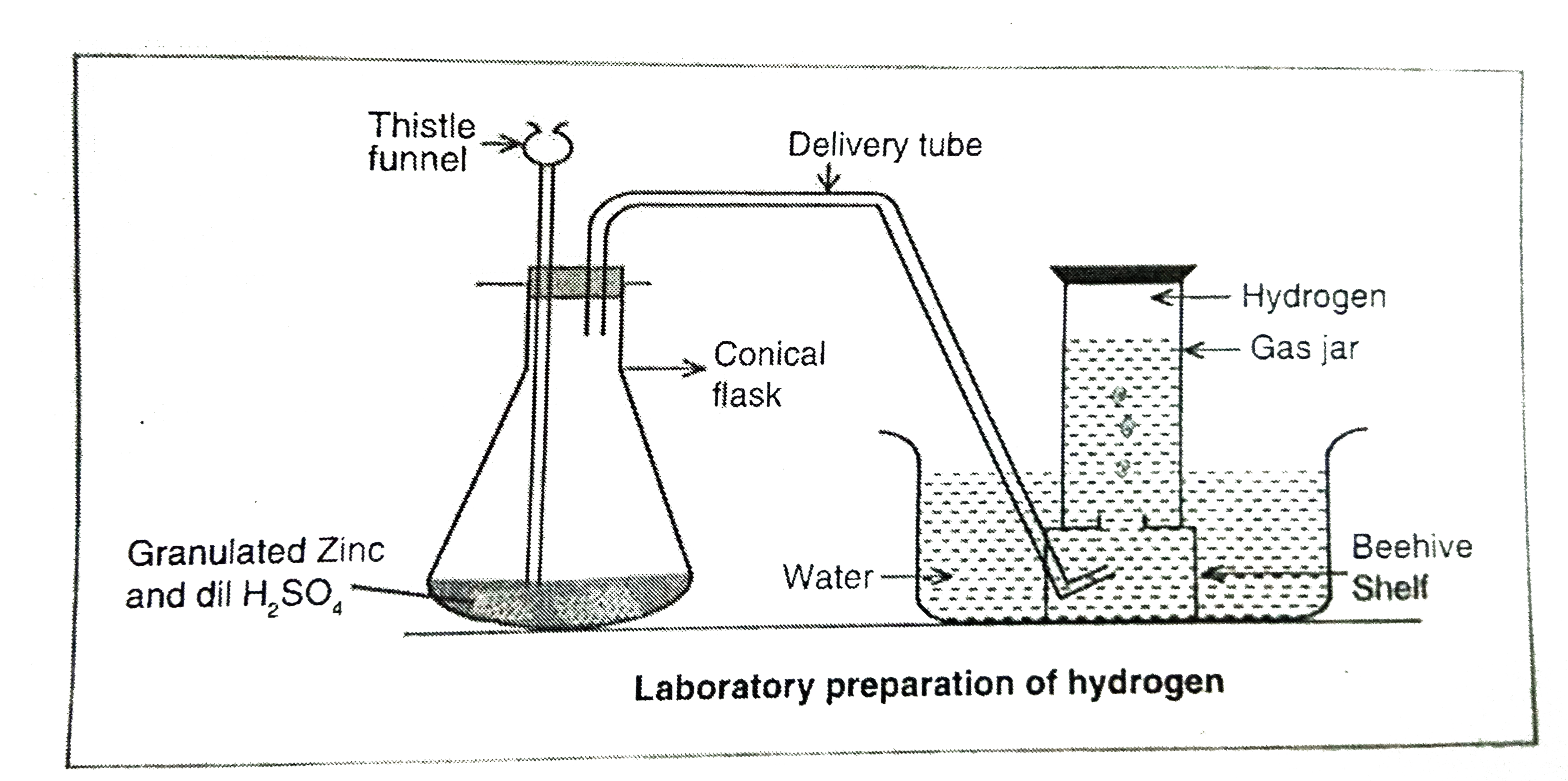 Experiment :Some granulated zinc pieces are taken in a flat-BOTTOM flask bottom flask fitted with a two holed air tigth cork. In one hole, a thistle a thistle funnel is inerted and in anoter hole, a long dilivery tube is inseted. Dilute `H_(2)SO_(4)` is added to the flask drop-wise through the thistile funnel. Observation: Effervescence is OBSERVED in the initial stages of the reaction. Thena colourless, and odourless gas evolves. Collection of gas: Hydrogen is collected by the downward DISPLACEMENT of water. |
|
| 40. |
Describe the chemical reaction of CO_(2) with (a) lime water (b) metals like sodium, potassium and calcium ( c) non-metals like carbon |
|
Answer» Solution :(a) Reaction with lime water `(Ca(OH)_(2))`: `CO_(2)` reacts with lime water that is `Ca(OH)_(2)` to form a milky white precipitate that is `CaCO_(3)`. `Ca(OH)_(2)toCaCO_(3)darr` When excess `CO_(2)` is passed through above solution, the presipitate dissolves due to the formation of `Ca(CHO_(3))_(2)` `CaCO_(3)+H_(2)O+CO_(2)TOCA(HCO_(3))_(2)` (b) Reaction with metals: Metals like Na, K and Ca react with `CO_(2)` to form correponding metal carbonates whils Mg and AI GIVE corresponding oxides. `4Na+3CO_(2)to2Na_(2)CO_(3)+C` `2Mg+CO_(2)to2MgO+C` (c ) Reaction with non-metals : `CO_(2)` when reats with red hot COKE reduced to CO , a very poisonous gas. `C+underset(("Coke"))(CO_(2))overset(Delta)(to)2CO` |
|
| 41. |
Describe briefly the major techniques applied on the molten glassobtained during the manufacture of glass to get the desired variety of glass. |
|
Answer» Solution :The major techniques applied on the molten GLASS obtained during the MANUFACTURE of glass to get thedesired variety of glass are : (i) Blowing of glass : This technique is used for the manufacture of laboratory glass ware, bottles ETC. (II) Optical glass: Molten glass is pressed into circular shape from which differentoptical glasses aremanufactured. (iii) SHEET glass: Molten glass obtainedfrom the glass tank furnace is placed on molten bath of tin. The molten glass spreads on the bath of molten tin withuniform thickness from which sheet glass/gloat glass is drawn. |
|
| 42. |
Derive the formula of the following compounds.(a)Ammonium nitrate(b)Ferrous sulphate |
|
Answer» Solution :(a)AMMONIUM nitrate `NH_(4)^(+)NO_(3)^(-)` `:.` Formulais `NH_(4)NO_(3)` (B)Ferrous sulphate `Fe^(+2)SO_(4)^(-2)` `:.`Formula is `FeSO_(4)` |
|
| 43. |
……….depletes the ozone layer. |
|
Answer» Chlorofluorocarbons DEPLETES the OZONE LAYER. |
|
| 44. |
Density of water is less than 1g/c.c ______ |
|
Answer» at `4^(@)C` |
|
| 45. |
Density of water is maximum |
|
Answer» at `0^(@)C` |
|
| 46. |
Define valency? |
| Answer» Solution :The numberof electrons gained or lost by an ATOM of an element forattaining STABLE octet or duplet configuration is called VALENCY. | |
| 47. |
Define the following and give an example each. (a) Efflorescent substances (b) Deliquescent salts (c) Hygroscopic salts |
|
Answer» Solution :(i) Efflorescent substances: The salts which lose water of crystallization either completely of PARTIALLY on exposure to atmospheric air are called efflorescent substances. `Ns_(2)CO_(3)*10H_(2)O` LOSES nine water MOLECULES on exposure to air and the remaining one water molecule on heating. (ii) Deliquescent substance: The substances which absorb moisture from the atmosphere on exposure to air. By absorbing moisture they may dissolve in the water absorbed and change into solution. Such substances are called deliquescent substances. Examples: CALCIUM chloride. (iii) Hygroscopic substances: The substances which do not undergo any change in physical state on the absorption of moisture from the atmospheric air. These are called hygroscopic substance. Examples: Calcium oxide, COBALT chloride etc. |
|
| 48. |
Define radical . Givesome examples of bivalent negative radicals. |
|
Answer» Solution :A group of ATOMS possessing either positive or NEGATIVE charge by losing or gaining one or more ELECTRONS is called a radical . The POSITIVELY charged radicals are ammonium radicals`(NH_(4)^(+))` and hydroium radical `(H_(3)O^(+))`. All other radicals arenegatively charged. `SO_(3)^(-2),SO_(4)^(-2),CO_(3)^(-2)`are some EXAMPLES of bivalent negative radicals. |
|
| 49. |
Define melting and boiling points. |
|
Answer» Solution :The temperature at which a solid CHANGES into a liquid on heating at normal atmosperic PRESSURE is called the MELTING point of that solid. The temperature at which conversion of liquid on heating at normal atmopheric pressure is liquid at normal atmosperic pressure is called the BOILING point of that liquid and the phenomenon is called boiling. |
|
| 50. |
Define cohesive and adhesive forces. |
|
Answer» Solution :The forces of ATTRACTION between the molecules of the same substance are known as COHESIVE forces WHEREAS adhsive forces are the forces which exist between the molecules of two different substances. For example, water particles stick to the glass ROD DUE to adhesive forces. |
|