Explore topic-wise InterviewSolutions in Current Affairs.
This section includes 7 InterviewSolutions, each offering curated multiple-choice questions to sharpen your Current Affairs knowledge and support exam preparation. Choose a topic below to get started.
| 1. |
Themetals with the least tensile strength is |
|
Answer» iron |
|
| 2. |
The metallic compounds of which among the following metals is involved is involved in cloud seeding ? |
|
Answer» Mercury |
|
| 3. |
The maximum number of electrons that can be accommodated in the outermost orbit is equal to _______. |
|
Answer» Eight is the maximum number of electrons that can be ACCOMMODATED in the VALENCE shell. |
|
| 4. |
The maximum number of electrons that can be accommodated in an orbit is equal to __________ where n is the number of orbits. |
|
Answer» `4n^(2)` |
|
| 5. |
The maximum number of electrons in L shell is two. |
|
Answer» SOLUTION :False The maximum number of ELECTRONS in L shell is eight. |
|
| 6. |
The mass of an electron is _______ times that of mass of a proton. |
|
Answer» The MASS of ELECTRONS is `(1)/(1837)` TIMES that of mass of a PROTON. |
|
| 7. |
The major component of naturalgas is _________ |
|
Answer» The major COMPONENT of natural gas is methane |
|
| 8. |
The inter conversion involved in usage of "odonil" in wash room is ________ . |
|
Answer» sublimation |
|
| 9. |
The increase in the proportion of ………… in air leads to global warming. |
|
Answer» CO |
|
| 10. |
The holes in the ozonosphere is due to the attack of a pollutant that is released from |
|
Answer» automobiles |
|
| 11. |
The formulae ofa compound when a positiveradical with valency 2 and nedical with valency 1 combine is _______. |
Answer» Solution :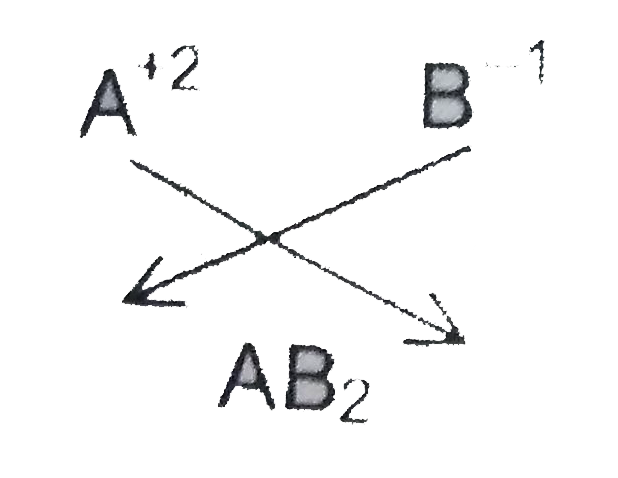
|
|
| 12. |
The conversion of a liquid to its solid state on cooling is called __________ . |
|
Answer» The process involving the CHANGE of from liquid to SOLID on cooling is called solidification. |
|
| 13. |
The compounds X and Y combine to form a compound Z. 'Z' which is need for construction of monuments gets affected by acid rain. The compound X is used in white washing. Identify X, Y and Z and write the corresponding balanced balanced chemical equations. |
|
Answer» SOLUTION :Since X is used in white washing, X is a `CA(HO)_(2)`, Y is a `CO_(2)` and Z is `CaCO_(3)` as it used for construction of monuments is `underset(X)(Ca(OH)_(2))+underset(Y)(CO_(2))tounderset(Z)(CaCO_(3))+H_(2)O` `CaCO_(3)+H_(2)O+CO_(2)toCa(HCO_(3))_(2)` |
|
| 14. |
The componenets of compound are separated by physical processes. |
|
Answer» Solution :False The CONSTITUENTS of a COMPOUND do not retain their properties and HENCE these cannot be SEPARATED by PHYSICAL methods. |
|
| 15. |
Thecombining capacity of the metal which can exhibit more than one valency is called___________. |
|
Answer» Certain metals likeFe, Cu , Sn, etc ., exhibit more than ONE valencu their compounds CALLED variable valency EXAMPLE :`FeCl_(2),FeCl_(3)` |
|
| 16. |
The colour change of CuSO_(4) solution can observed when it is made to react with |
|
Answer» silver |
|
| 17. |
The CO that is added up to the atmosphere due to the incomplete combustion of fuels can be decreased by the |
|
Answer» usage of scrubber |
|
| 18. |
The chemical name of sand is |
|
Answer» silicon |
|
| 19. |
The chemical formula of rust is ………… |
|
Answer» The CHEMICAL formula of rust is `Fe_(2)O_(3).XH_(2)O`. |
|
| 20. |
The chemical formula of plaster of Paris is _________ |
|
Answer» `CaSO_(4).1//2H_(2)O` The chemical FORMULA of plaster of Paris is `CaSO_(4).1//2H_(2)O`. |
|
| 21. |
The chemical composition of limestone is |
|
Answer» CALCIUM carbonate |
|
| 22. |
The charge possessed by permanganate is the same as ___________ radical. |
|
Answer» magnesium |
|
| 23. |
The change of water into water vapour at 30^(@)C at slow rate is called ______. |
|
Answer» |
|
| 24. |
The central part of the atom called nucleus accounts for the mass of an atom. |
|
Answer» Nucleus CONSISTS of both PROTONS and neutrons and it ACCOUNTS for the mass of the ATOM. |
|
| 25. |
The atomicity of which among the following is the maximum ? |
|
Answer» Helium |
|
| 26. |
The atomic numbers of the elements X, Y and Z are 2, 10 and 16, respectively. Identify the element which does not exist in monoatomic state. |
|
Answer» X SULPHUR ( S)doesn ' t exist in monoatomic state due to its greater chemical reactivity. |
|
| 27. |
The atomic number of three elements A, B and C are 10 , 18and 8respectively . Identify the element which is chemically reactive ? |
|
Answer» A HENCE , 'C' is chemically reactive . A and B are chemiclly INERT because of stable octet configuration. |
|
| 28. |
The atomic number and mass number of an element are 13 and 27 respectively. Find the number of neutrons. |
| Answer» SOLUTION :NUMBER of neutrons=27-13 =14 | |
| 29. |
The atmosperic pressure is maxmum at ……… and found to be equal to ……… |
|
Answer» |
|
| 30. |
The amount of heat energy required to increase the temperature of X g of water by 10^(@)C is found to be 15 cal. Calculate X. |
|
Answer» 0.5 g `therefore` Heat REQUIRED for increasing `10^(@)C` temperature of X g water is 10 X cal. `therefore` 10X = 15 `therefore` X = 1.5 g |
|
| 31. |
The amount of heat energy required to increase the temperature of 'X' g of water by 10 .^(@)C is found to be 15 cal. Calculate 'X'. |
| Answer» Solution :The SPECIFIC HEAT of water is 1 cal `g^(-1) .^(@)C^(-1)`. Therefore the amount of heat required is 1.5 cal for 15 for `10^(@)C` raise in temperature. | |
| 32. |
The amount of heat energy required to increase the temperature of 20 g of water by 1^(@)C is ______. |
|
Answer» 10 cal Amount of heat ENERGY REQUIRED is `20 xx 1` = 20 cal. 1 cal - 1 g ? - 20 g = 20 cal |
|
| 33. |
The …………. Alters the rate of reaction |
|
Answer» The catalyst ALTERS the RATE of REATION. |
|
| 34. |
Tellurium shows the properties of both metals and non-metals. |
|
Answer» Tellurium is a metalloid and HENCE it show PROPERTIES of both metals and nonmetals. |
|
| 35. |
Tartaric acid is one of the components in baking powder. What is its role? |
| Answer» SOLUTION :During BAKING, baking SODA present in baking powder induces bitter taste and YELLOW colour to breads and cakes due to the alkaline nature of its aqueous solution. To neutralize bitter taste of alkaline taste and to remove yellow colour, tartaricacid is USED. | |
| 36. |
Sunny and Bunny are neighbours staying in the same apartment. In summer vacation, one day they wanted to prepare some snaks for eating. During that process, the food material fell on the floorand the beautiful marble flooring became dirty. Sunny was afraid that his mother would scold him and in aa hurry, brought some acid to clean the floor. But, Bunny stopped him and said that it will spoil the flooring permanently. Why did Bunny say so? Give reason. |
| Answer» Solution :The major component of marble is `CaCO_(3)`. It being BASIC in NATURE, REACTS with an acid to FORM salt and water (neutralisation REACTION). Hence acids being reactive with marble, they cannot be used for cleaning marble flooring. | |
| 37. |
Sulphur dioxide on hydrolysis gives _________. |
|
Answer» |
|
| 38. |
Sugar gets charred when treated with conc. H_(2)SO_(4) . This is an example for __________ |
|
Answer» physicalchange. `C_(12)H_(22)O_(11)overset("conc."H_(2)SO_(4))to12 C+11H_(2)O` |
|
| 39. |
Sublimation of camphor is a physical change. |
|
Answer» Sublimation of CAMPHOR is a PHYSICAL CHANGE. |
|
| 40. |
Sublimation is the process of the conversion of a solid to its liquid state. |
|
Answer» SOLUTION :FALSE Sublimation is the PROCESS of the CONVERSION of a solid to its GASEOUS state. |
|
| 41. |
Sublimation is involved in _________ . |
|
Answer» INCENSE stick and odonil. |
|
| 42. |
State and explain the anomalous expansion of water. |
| Answer» Solution :In GENERAL, solids posses greater densities than liquids. Therefore, for such liquids, the density DECREASES with an increase in temperature as the liquids expand on heating. However, in the case of water, it is different. Ice has lower density than water at the same temperature. As ice gets converted to water from `0^(@)C` its density increases and this trend continues upto `4^(@)C` temperature. Beyond `4^(@)C`, water shows NORMAL trend of decreases in density with increase in temperature. Therefore, density of water is maximum at `4^(@)C` (1g/cc). From `4^(@)C` to `0^(@)C`, water undergoes expansion while all other liquids undergo CONTRACTION. Since this trend is opposite to the normal trend, this is called anomalous expansion of water. | |
| 43. |
Spring water usually contains |
|
Answer» dissolved salts |
|
| 44. |
Specific heat of water is 1 cal g^(-1) .^(@)C^(-1) |
|
Answer» |
|
| 47. |
Sodium floats on water. |
|
Answer» As the density of SODIUN is less than that of WATER it FLOATS on water. |
|
| 48. |
Sodium catches fire easily and chlorine is a harmful gas. But sodium shloride is indispensable in our daily meal. Give reasons. |
| Answer» Solution :SODIUM CHLORIDE is a compound. In compound, the constituents (sodium,chlorine) do not retain their properties and so we can TAKE NaCl in our DAILY MEAL. | |
| 49. |
Soda water contains excess……… in dissolved state. |
|
Answer» `H_(2)` |
|