Explore topic-wise InterviewSolutions in Current Affairs.
This section includes 7 InterviewSolutions, each offering curated multiple-choice questions to sharpen your Current Affairs knowledge and support exam preparation. Choose a topic below to get started.
| 1. |
Ina science fair , a student took two test tubes labelled A and B filled with copper sulphate solution, then he put magnesium ribbon in test tube A silver foil in tube B.Predict the observation and justify. |
|
Answer» SOLUTION :`{:("A","B"),(CuSO_(4),CuSO_(4)),(+,+),(Mg,Ag),(darr,darr),(MgSO_(4)+Cu,"No reaction"):}` The blue coloured `CuSO_(4)`solution in test tube 'A'disappears and thus COLOURLESS sloution of`MgSO_(4)`is formed .thisis due to higher reactivity of Mg than Cu . Hence Mg can DISPLACE Cu from `CuSO_(4)`solution and hence blue coloured `CuSO_(4)` SLOWLY turns colourless .Ag being less reactive than Cu, cannot displace Cufrom`CuSO_(4)` and hence the solution remains blue. |
|
| 2. |
In a ________ properties of the constituents are retained. |
|
Answer» In a mixture the PROPERTIES of its CONSTITUENTS are RETAINED. |
|
| 3. |
In a double decomposition reaction , one of the products is insoluble . Whattypeof reaction is that ? |
| Answer» SOLUTION :It is CALLED PRECIPITATION REACTION. | |
| 4. |
In a chemistry laboratory, aresearch scholar while conducting an experiment , observed at a certain step, that a yellow coloured gas (X) was released and it has been found to bepoisonous. This was later made to react with a highly reactive metal (Y). A new compound (Z)wasformed which is used for preservation of fish, meat and pickles. The professor asked him toidentify X, Y and Z. |
| Answer» SOLUTION :A greenish YELLOW POISONOUS gas 'X' is CHLORINE. Onreaction with a metal, sodium (highly REACTIVE metals) chlorine gas forms sodium chloride which is used for preservation of fish, meats and pickles. So 'Z' is sodium chloride, 'X' is chlorine gas and Y is sodium metal. | |
| 5. |
Ina chemical laboratory , a student broke the thermometer while performing an experiment . Immediately, the mercury which was spilled over was removedby the lab - incharge by sprinkling sulphur on it . Theteacher asked the students to predict the type of change / reaction associated with the above process. Explain with apporpriate reasons. |
|
Answer» Solution :When mercury gets spilled on the floor or any surface , it can be collected by SPRINKLING sulphur powder because HG reacts with S to form HGS. `Hg+StoHgS` It is element - element combination that is SYNTHESIS reaction. |
|
| 6. |
If theformula of respective chlorides of X andY are XCl_(3) andYcl_(4) respectively , then the valencies of X and Y are |
|
Answer» 3 and 2 |
|
| 7. |
If the formula of metal sulphite of a metal M is MSO_(3) ,give the formulae of its(a)phosphate(b)hydrogen phosphate (c ) chloride |
Answer» Solution :In`MSO_(3),`the valency of sulphite is 2. HENCE the valency of M is also 2.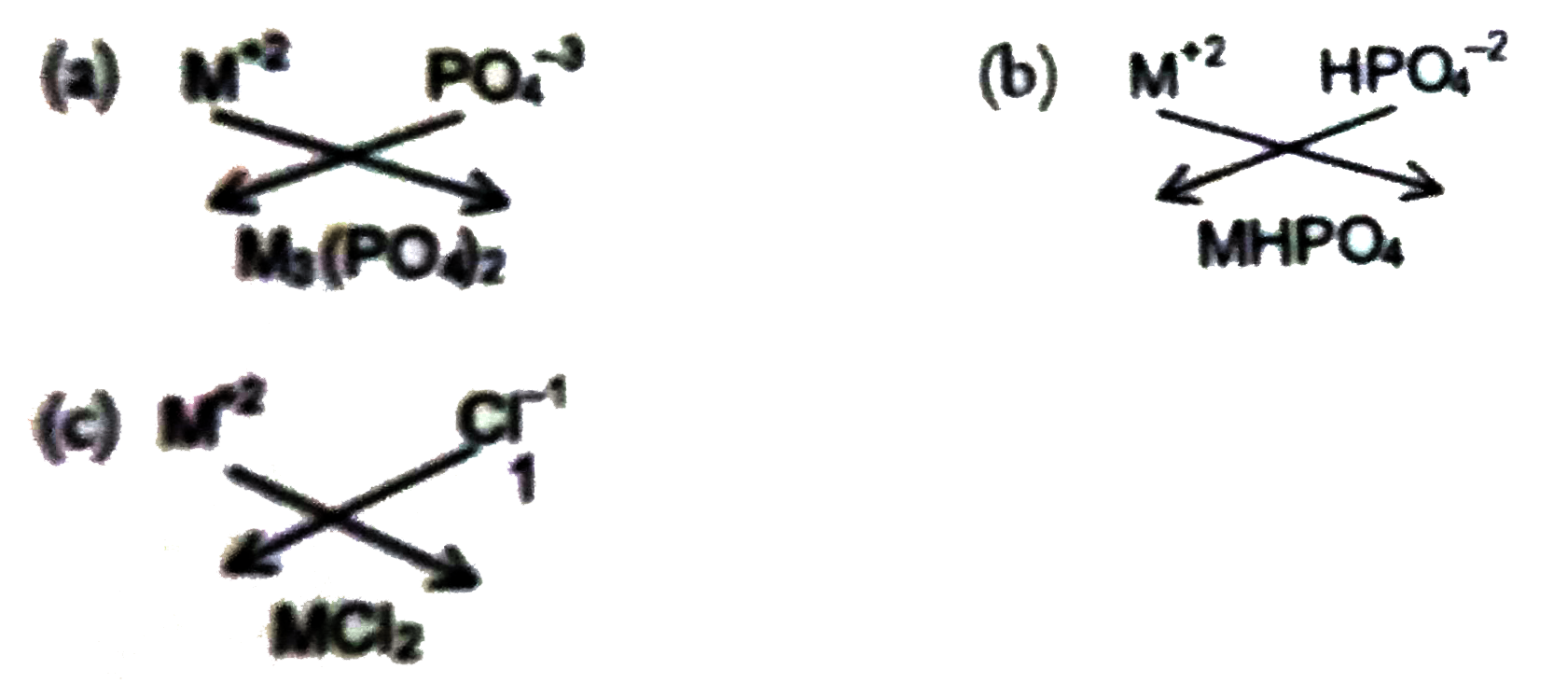
|
|
| 8. |
If the formula of a metal ion is M^(+2)then the formula of its dihydrogen phosphate is |
|
Answer» `M_(2)(PO_(4))_(3)` 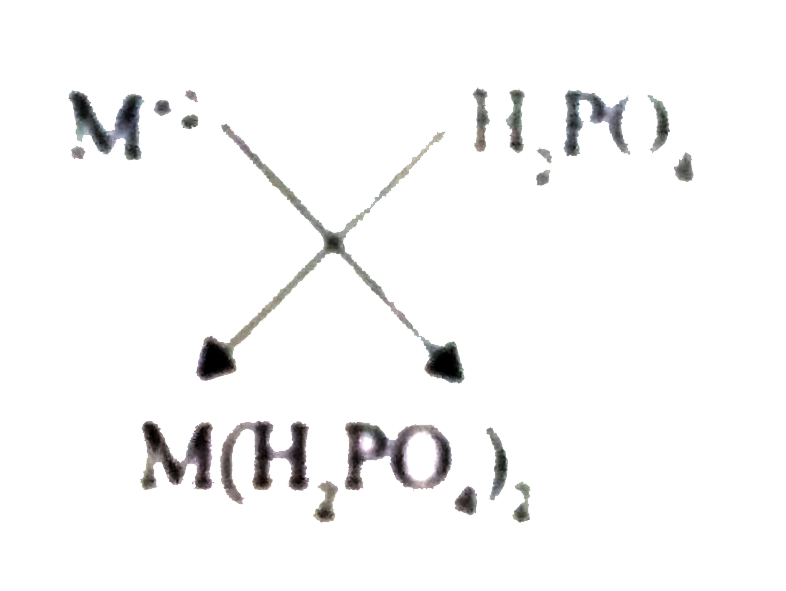
|
|
| 9. |
If the formula of a metal nitrite is M(NO_(2))_(2.), then the formula of its dihydrogen phosphate is |
|
Answer» `M_(2)(PO_(4))_(3)` 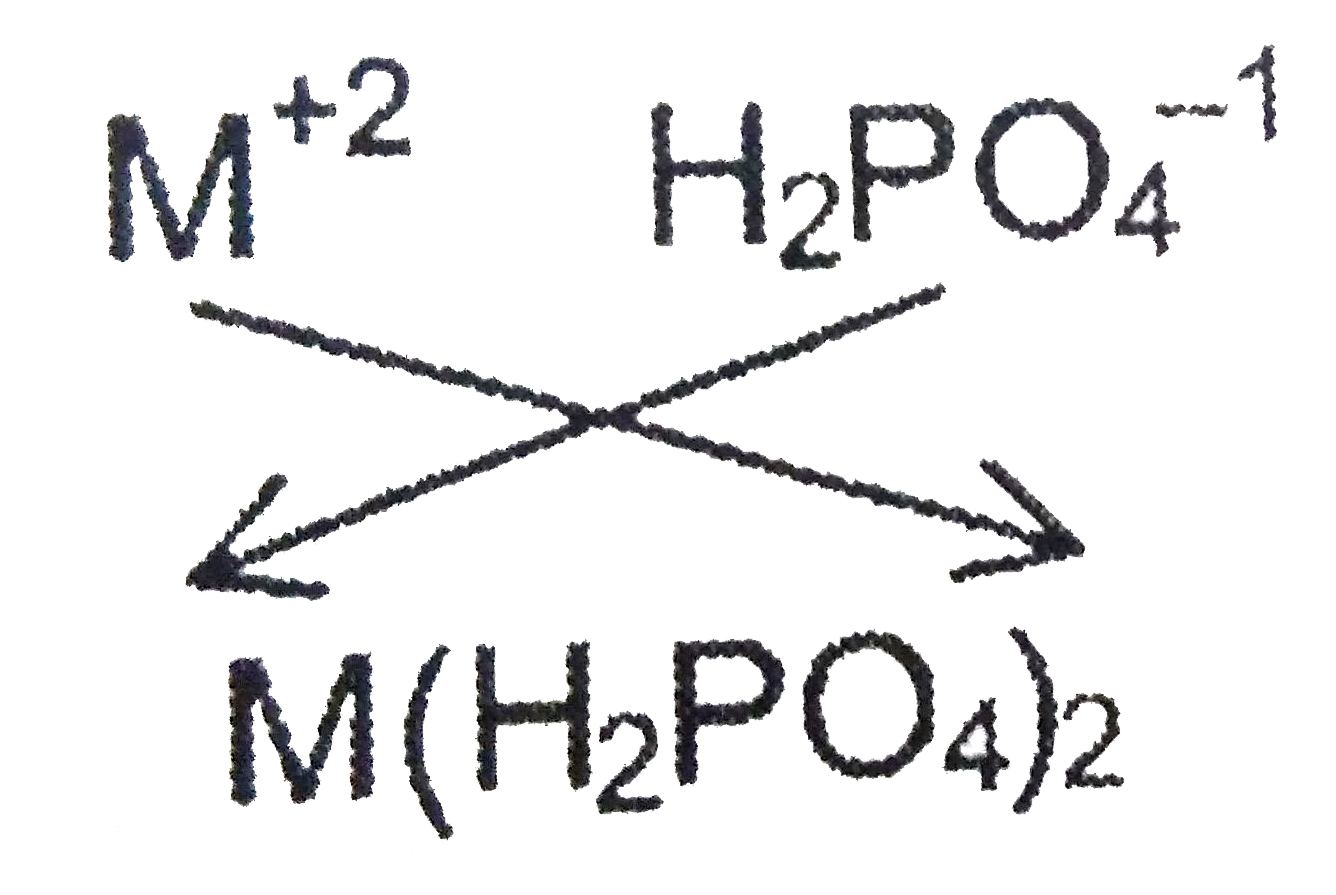
|
|
| 10. |
If overline(X) ion has 10 neutrons and 9 protons , then the electronic configuration of the atom of the element x is |
|
Answer» 1 ,8 ,1 `Xoverset(+l e^(-1))toX^(-1)` (9 electrons)""(10 electrons) Therefore , the electronic CONFIGURATION of an atom is 2 , 7 |
|
| 11. |
If a uninegative ion has 10 neutrons and9 protons, electronicconfiguration of the atom ofthe element is |
|
Answer» Solution : `X^(-) = 9` PROTONS (Z) 10 neutrons 10 electrons `Xoverset(+1e^(-))toX^(-1)`(9 electrons)""(10 electrons) Therefore , the electronic configuration of an atom is 2 , 7 |
|
| 12. |
If a solidnon- metal 'X'forms oxide of type X2O5 , the valency of X is |
|
Answer» 3 Valency of NON - METAL is 5. |
|
| 13. |
If a solid non-metal X forms oxide of type X_(2)O_(5),then the formula of its corresponding chloride is ___________ . |
|
Answer» `XCl_(3)` SINCE the valency of chloride ION is 1, the FORMULA of the aobve chloridewould be 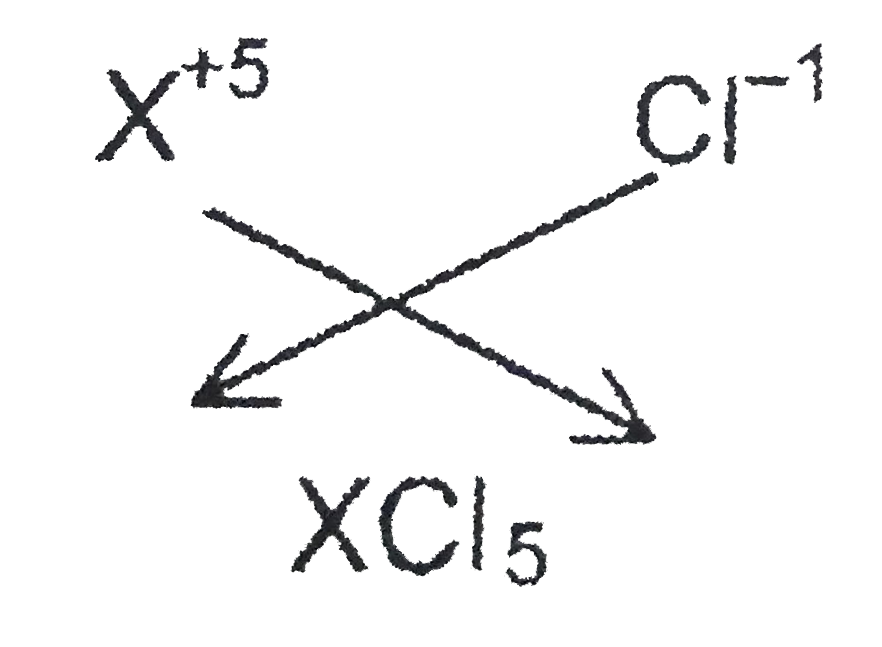
|
|
| 14. |
If a nonmetal forms only two oxides and one oxide on hydrolysis gives an acid, then the non-metal could be |
|
Answer» sulphur `CO_(2) + H_(2)O rarr H_(2)CO_(3)` |
|
| 15. |
If a dipositve ion of an element 'X' consists of 12 protins and 12 neutrons and another dipositve ion of an element 'Y' consists of 18 electrons and twice the number of electrons of dipositive ion X is the number of neutrons of Y . Thenthe mass numbers wouldbe in the ratio of |
|
Answer» Solution :In`X^(+2)`, Number of protons = 12 (Z) NEUTRONS = 12 Number of electrons = 10 Mass number (A) = 12 + 12 = 24 In `Y^(+2)` electrons are there. In Y 20 electrons or 20 protons (Z) are there . Mass number (A) = 20 +20 = 40 `:.`Ratio of mass number`=A_(x) "" : "" A_(y)` `{:(24,:,40),(3,:,5):}` |
|
| 16. |
Ifadispositve ion of an element 'X' consists of 12 protons and 12 neutrons and another dipositve ion of an element 'Y' consistsof 18 electrons and twice the number of electrons of dipostive ion X is the number of neutrons of Y. Then the mass number would be in the ratio of |
|
Answer» `1:2` NEUTRONS= 12 No . Of electrons= 10 A= 12+ 12=24 `Y^(+2)` =18 electrons. Y = 20electrons or 20 protons (Z) A=20+20= 40 `A_(X):A_(Y)` `24:40` `3:5` |
|
| 17. |
Identify true statement among the following. |
|
Answer» The CONSTITUENTS of both compound and mixture can be separated by PHYSICAL methods only. |
|
| 18. |
Identify the wrong statement among the following. |
|
Answer» MOLECULES of SOLIDS prossesss only vibratory motion. |
|
| 19. |
Identify the true statement among the following. |
|
Answer» Both COMPOUNDS and mixtures are HOMOGENEOUS. |
|
| 20. |
Identify the substance which continue to burn in an atmosphere ofN_(2). |
|
Answer» Fe |
|
| 21. |
Identify the substance which doesn't continue to burn in an atmosphere of H_(2). |
|
Answer» Na |
|
| 22. |
Identify the strong acid and weak base among the following respectively. |
|
Answer» HCI and KOH |
|
| 23. |
Identify the soft metal among the following |
|
Answer» IRON |
|
| 24. |
Identify the set of pure and impure water among the following |
|
Answer» RAIN water, sea water |
|
| 25. |
Identify the salt which does not contain either replaceable hydrogen ions or hydroxyl ions. |
|
Answer» Magnesium hydroxy chloride |
|
| 26. |
Identify the ratio of the coefficients of the products CuO,NO_(2) and H_(2)Oformed respectively when hydrated copper nitrate is thermally decomposed.2Cu(NO_(3))_(2).6H_(2)OtoNO_(2)+H_(2)O+O_(2)+2CuO |
|
Answer» `2:3:1` Coefficients of CuO, `NO_(2) and H_(2)O` are RESPECTIVELY 2 , 4AND 6. `:.` RATIO is`1:2:3` |
|
| 27. |
Identify the pair of water soluble bases. |
|
Answer» Copper oxide, potassium oxide |
|
| 28. |
Identify the organic acid among the following given acids. |
|
Answer» Hydrochloric ACID |
|
| 29. |
Identify the odd one among the following with respect to tensile strength as well as ductility. |
|
Answer» GAS CARBON |
|
| 30. |
Identify the number ofneutrons present in a pair of element with atomic numbers 20 and 13 , if their mass numbers are 40 and 27 respectively. |
|
Answer» 20 and 13 (1)40 - 20 = 20 (2)27 - 13 = 14 |
|
| 31. |
Identify the number of neutrons present in a pair of elements with atomic numbers 20 and 13, if their mass numbers are 40 and 27, respectively. |
|
Answer» 20 and 13 (1)40 - 20 =20 (2) 27 - 13 =14 |
|
| 32. |
Identify the layers of atmosphere as per activites mentioned and arrange them in sequence. Burning up of meteorites, flying of aeroplances, rflection of radio waves, absorption of harmful ionizing radiations and occurrence of most of the weather phenomena. (a) Troposphere (b) Stratophere ( c) Mesosphere (d) Ionophere (e ) Megnetosphere |
|
Answer» bcdea |
|
| 33. |
Identify the four layers of atmosphere as per the temperature range mentioned and them in a sequence. 60^(@)C" to "-15^(@)C" to "17^(@)C" to "-51^(@)C,-15^(@)C" to "-120^(@)C and maximizing up to 2000^(@)C. (a) Troposphere (b) Stratosphere ( c) Mesosphere (d) Exosphere ( e) Thermosphere |
|
Answer» BACE |
|
| 34. |
Identify the formula of the corresponding hydrideof a nonmetal 'X' which attainsoctet by gaining three electrons. |
|
Answer» XH Hence , its corresponding hydride is`X^(-3)H^(+1)rArrXH_(3)` |
|
| 35. |
Identify the formula of the corresponding hydrideof a nonmetal 'X' which attainsoctet by gaining twoelectrons. |
|
Answer» XH |
|
| 36. |
Identify the false statement among the following. |
|
Answer» Compound is homogeneous in nature. |
|
| 37. |
Identifythe components which when present in the troposphere is a pollutant and when present in upper layers of atmosphere is a protector of life. |
|
Answer» Chlorofluro carbons Hence the ozone layer of the STRATOSPHERE acts as a shield. |
|
| 38. |
Identify the common use(s) of both chile salt petre and nitre. |
|
Answer» Manufacture of gunpowder. |
|
| 39. |
Identify the coefficients of the product CuO andNO2formed respectively when hydrated copper nitrate is themally decomposed.2Cu(NO_(3))_(2).6H_(2)OtoCuO+NO_(2)+6H_(2)O+O_(2) |
|
Answer» Solution :`2Cu(NO_(3))_(2)*6H_(2)Ooverset(Delta)to2CuO+4NO_(2)+O_(2)+6H_(2)O` Coefficients of`CuOand NO_(2)`are respectively 2 and 4. |
|
| 40. |
Identify the atomic numbers of the pair of elements whichpossessessame number of electrons in L and M shells. |
|
Answer» 12 , 18 |
|
| 41. |
Identify the atomic number of the element which possess same number of electrons in L and M shells. |
|
Answer» 18 |
|
| 42. |
Identify the acid used in the purification of metals like gold and silver is |
|
Answer» sulphuric ACID |
|
| 43. |
Identify physical and chemical changesamong the following.(a) Boiling of milkRipening of fruits(c ) Curding of milk(d)Melting of candle(e ) Rusting of iron(f) Blackening of silverware |
| Answer» SOLUTION :`{:("Physical change ","Chemicalchange"),("Boiling of milk , melting of CANDLE","RIPENING of fruits , curdling of milk , RUSTING if iron, blackening of SILVERWARE"):}` | |
| 44. |
Identify a useful change among the following chemical changes. |
|
Answer» Blackening of silverware |
|
| 45. |
Identify a false statement among the following. |
|
Answer» Evaporation is a surface phenomenon and CAUSES cooling. |
|
| 46. |
Identifty the xomponent which when present in the troposphere is a pollutant and when present in upper layers of atmosphereis protector of life. |
|
Answer» Chloroflurocarbons |
|
| 47. |
(i) What is the composition of pencil leads? (ii) How are crucibles made with the help of graphite? (iii) How are crucibles made with the help of graphite? (iv) Why is graphite a more preferred lubricant to the ordinary lubricating oils? (v) What is the role of graphite in nuclear reactor? |
|
Answer» Solution :(i) Pencil leads are manufactured by mixing graphite with CLAY. (II) The more theamount of clay, the harder is thepencil. (iii) Graphite mixed with FINE clay is moulded and sintered into crucibles of desired size and shape. (iv) Graphite is highly RESISTANT to heat andtherefore used as lubricant in those parts of machinery where HIGH heat is generated due to friction. (v) Graphite is used in nuclear reactors as a moderator to slow down the speed of nueutrons produced due to splitting of theatoms of uranium or thorium. |
|
| 48. |
(i) What are hydrocarbons? (ii) Give the formula of the simplest hydrocarbon. (iii) What is bio gas? (iv) Give two advantages of bio gas. (v) Name the products obtained on burning methane gas. |
|
Answer» Solution :(i) The ORGANIC compounds which contain only carbon and hydrogenare called hydrocarbon. (II) THEFORMULA of simplest hydrocarbon is `CH_(4)`. (iii) The DECOMPOSITION of farm waste and cow dung in the absence of air gives a mixture of gases called bio gas. (iv) (a) It is pollution free. (b) It has high ratio of energy given out by combustion as compared to the other fuels. (v) Carbon dioxide andwater VAPOUR are theproducts formed on burning methane gas. |
|
| 49. |
(i) Name the metal which is used in the manufacture of high voltage electric transmission wire. (ii) Why is aluminium used in the manufacture of household articles? (iii) Write the name and the formual of the monomer of polyethylene (iv) Among which alloys the percentage of zinc is less (Brass/Bronze)? (v) Why is silicone used in the manufacture of nonstick pans? |
|
Answer» Solution :(i) Aluminium metal is used in the manufacture of high voltage electric transmission wire. (II) Aluminium is corrosion resistant due to surface oxide formation . Hence, used in the manufacture of HOUSEHOLD articles. (iii) Monomer of polyethylene is ETHYLENE molecule having MOLECULAR formula ` C_(2)H_(4)`. (iv) Bronze alloy has less percentage of zinc. (v) Silicone is highly heat resistant, non-sticky and water REPELLENT. |
|
| 50. |
(i) Name the inert gas which is used for filling weather observation balloons. (ii) Why is Radon used in cancer treatment ? (iii) Why is an alloy of tin and lead used in the manufacture of fuse wire? (iv) Name thegas which is used in advartising sign boards. (v) Why is argon used in filling electric bulbs? |
|
Answer» Solution :(i) Helium (II) Radon is radioactive element. (iii) TIN and lead have lower melting points than other merals. (iv) Neon (v) Argon is filled in ELECTRIC bulbs to improve the efficiency of bulb by preventing OXIDATION of tungsten FILAMENT. |
|