Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 21151. |
Which of the following is a hypervalent compound? |
|
Answer» `BF_3` |
|
| 21152. |
Which of the following will show a negative deviation from Raoult's law ? |
|
Answer» ACETONE - BENZENE |
|
| 21153. |
What happens when PH_(3) reacts with oxygen or air? |
|
Answer» <P> SOLUTION :When phosphine air or OXYGEN it burns to give meta phosphoric acid.`4PH_(3)+8O_(2)overset(Delta)rarr underset("(Phosphorous pentoxide)")(P_(4)O_(10)+6H_(2)O)` `P_(4)O_(10)+6H_(2)underset("Meta phosphoric acid")(overset(Delta)rarr4HPO_(3))+4H_(2)O` |
|
| 21154. |
Which of the following will be the most stable diazonium salt(RN_(2)^(+) X^(-)) ? |
|
Answer» `CH_3N_2^(+)X^(-)` |
|
| 21155. |
The substance which is not an artificial sweetener |
|
Answer» sucralose |
|
| 21156. |
Which of the following ions is diamagnetic? |
|
Answer» `[Ni(H_(2)O)_(6)]^(2+)` 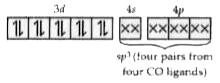 CO is a strong field and in `[Ni(CO)_(4))` complex AL the ELECTRONS in d-orital are paired. Hence `[NI(CO)_(4)]` is diamagnetic in nature. `H_(2)O` & `Cl^(-)` are weak field ligand hence do noc cause paireing. |
|
| 21157. |
Which one of the following statements is not true regarding (+) lactose |
|
Answer» (+)LACTOSE, `C_(12)H_(22)O_(11)` CONTAINS 8-OH groups |
|
| 21158. |
The volume of water to be added to 100 mL of 0.5N H_(2)SO_(4) acid solution to get solution of decinormal concentration is : |
|
Answer» 400 mL `(0.5 N)xx(100mL)=(0.1 N)xxV_(2)` `V_(2)=((0.5N)xx(100mL))/((0.1N))=500mL` Volume of water to be added=(500-100)=400 mL |
|
| 21159. |
What is denaturaedalcohol? |
| Answer» SOLUTION :ALCOHOL is MADE unfit for drinking by mixing some copper SULPHATE and pyridine. This is called denatured alcohol. | |
| 21160. |
The standard enthalpy of neutralization of strong acid and strong base is -57.3 kJ "equiv"^(-1). If the enthalpy of neutralization of the first proton of aqueous H_(2)S is -33.7 kJ mol^(-1) then the (pK_a)_(1) of H_(2)S is |
|
Answer» `((23.6 XX 10^(3) - T Delta s^@)/(2.303 RT))` |
|
| 21161. |
When aqueous solutions of two salts are mixed , the third salt formed may appear as a solid precipitate or a clear solution depending upon the solubility of its ions . It is observed that all salts of Na , K , NH_(4) are soluble . All nitrates and bicarbonates are also soluble . All halides (chlorides , bromides , iodides) are soluble except those of Ag , Hg (I) and Pb . Which one among the following combinations of solutions will produce a solid precipitate ? |
|
Answer» Sodium sulphate and barium chloride `underset("sulphate")underset("Sodium") (Na_(2)SO_(4)) + underset("chloride")underset("Barium")(BaCl_(2)) to underset("(SOLUBLE)")(2NaCl) + underset(("ppt"))(BaSO_(4)) darr` `underset("sulphate")underset("Magnesium")(MgSO_(4)) + underset("bicarbonate")underset("Barium")(Ba(HCO_(3))_(2)) to Mg underset(("Soluble"))((HCO_(3))_(2)) + underset((ppt))(BaSO_(4)) darr` Both the above given combinations of solution will produce a precipitate of `BaSO_(4)`. Hence , option (a) white and (b) both are correct ANSWER. |
|
| 21162. |
Write structures of the following compounds. (i) 2. Chloro - 3 - methylpentane (ii) 1. Chloro - 4-ethylecyclohexane (iii) 4. tert. Butyl - 3 - iodoheptane |
|
Answer» SOLUTION :(iv) `BrCH_(2)CH = CHCH_(2)Br` (v) 
|
|
| 21163. |
Values of heats of formation for SiO_(2) and MgO are -48.4 and -34.7 kJ respectively. The heat of the reaction 2Mg+SiO_(2)rarr2MgO+Si is |
|
Answer» 21.16 kJ `=2(-34.7)-(-48.4)=-21 kJ`. |
|
| 21164. |
Which one of the following products obtained when diethyl when diethyl ether is boiled with water in presence of dilute acid |
|
Answer» Glycol `R-O-R+H_(2)Ooverset("dil. H_(2)SO_(4))to 2R-OH` |
|
| 21165. |
Which of the following behaviour is true about the ideal binary liquid solution? |
|
Answer» <P>PLOT of P(total) against `x_(A)` is non linear . |
|
| 21166. |
Which of the following compound does not show metamerism? |
|
Answer» `CH_3-O-CH_3` |
|
| 21167. |
When AgNO_(3) solution is added in excess to 1 M solution of CoCl_(3).XNH_(3) one mole of AgCl is formed? What is the value of 'X' ? (Assume that the co - ordination number is 6) |
|
Answer» 1 |
|
| 21168. |
Which of the following cannot be used as alkylating reagent in Friedel-Crafts reaction- |
|
Answer» `(CH_3)_2CHCl` |
|
| 21169. |
Which base is present in place of thiamine in RNA ? |
|
Answer» URACIL |
|
| 21170. |
Which among the following group 16 elements exists in more than two allotropic states ? |
|
Answer» POLONIUM 
|
|
| 21171. |
What are secondary cells? |
| Answer» SOLUTION :Secondary CELLS are those which can be recharged by passing electric current through them and HENCE can be USED over and again. | |
| 21172. |
Which of the following is an oxidising agent? |
|
Answer» `[Mn_(2)(CO)_(10)]` |
|
| 21173. |
Which of the following has more atomic size |
|
Answer» N |
|
| 21174. |
Which of the following hormones helps in the conversion of glucose into glycogen in the body: |
|
Answer» Insulin |
|
| 21175. |
What us SHE ? |
|
Answer» |
|
| 21176. |
Which of the following compound not shows test of unsaturation :- |
|
Answer»
|
|
| 21177. |
What type of a battery is the lead storage battery 7 Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery when current is drawn from it. (b) In the button cell, widely used in watches, the following reaction takes place : Zn(s) + Ag_(2)O (s) + H_(2)O (l) to Zn^(2+)(aq) + 2Ag (s) + 2OH^(-) (aq) Determine E^(@) and DeltaG^(@) for the reaction. [Given: E_(Ag^(+)//Ag)^(@) =+0.80 V, E_(Zn^(2+)//Zn)^(@) =-0.76 V] |
|
Answer» Solution :Lead storage battery is a secondary cell. Reactions at the anode and cathode when the current is drawn from it (discharge) are as follows : At anode: `Pb(s) + SO_(4)^(2-)to PbSO_(4)(s) + 2e^(-)` At cathode: `PbO_(2) (s) + 4H^(+)+ SO_(4)^(2-) to PbSO_(4) (s) + 2H_(2)O(L)` Overall reaction: `Pb(s) + PbO_(2) (s) + 2H_(2)SO_(4) (AQ) to 2PbSO_(4) + 2H_(2)O(l)` (b) `Zn(s) + Ag_(2)O(s) + H_(2)O (l) to Zn^(2+) (aq) + 2Ag(s) + 2OH^(-) (aq)` GIVEN: `E_(Ag^(+)//Ag)^(@) = +0.80 V, E_(Zn^(2+)//Zn)^(@) = -0.76 V` `E_("cell")^(@) = E_("cathode")^(@) -E_("anode")^(@)` `=0.80 =-nFE^(@)` `=-2 xx 96500 C mol^(-1) xx 1.56 V = -30180 J mol^(-1)` `=-301.080 kJ mol^(-1)` |
|
| 21178. |
What is native state of protein ? |
| Answer» Solution :The ENERGETICALLY most STABLE shape of the protein at NORMAL pH and temperature is called native state. | |
| 21179. |
The rustingof iron takesplace as follows 2H^(opluse)+2e^(-)+1/2 O_(2)rarrH_(2)O(l),E^(-)=+1.23 V Fe^(2+)(aq)+2e^(-)+2e^(-)rarrFe(S),E^(-1)=-0.44V triangleG^(-) forthe netprocess is |
|
Answer» `-322 kJ mol^(-1)` At ANODE `Fe(s) rarr Fe^(2+) rarr Fe^(+) (aq) +2e^(-)` `triangle G_(2)^(-) =- 2xxFe^(-) =(-) 2xxF(0.44)` `2H^(+opluse)(aq)+1/2 O_(2) +Fe(s)rarrH_(2)O(l)+Fe^(2+) (aq)` `triangle G_("net")^(-) =[-2F(1.123 )] +(-) 2F(0.44) ] =- 322.3 kJ "mol"^(-)` |
|
| 21180. |
Write the chemical equations involved in the preparation of the following: (a) XeF_4(b) H_3PO_3 |
|
Answer» SOLUTION :Chemical involved in the PREPARATION of `XeF_4` and `H_3PO_3` are given below : (a) `XE + 2F_2 underset("1 BAR ") OVERSET(873K) to XeF_4` (b) `underset(1:5 " ratio " ) (P_4O_6 ) + 6H_2O to 4H_3PO_3` (Phosphorous acid ) |
|
| 21181. |
Write the valence shell electronic configuration group - 16 elements. |
| Answer» SOLUTION :`NS^(2)NP^(4)` | |
| 21182. |
The solubility products of MA, MB,MC and MD are 1.8 xx 10^(-10) , 4 xx 10^(-3) , 4 xx 10^(-8)and 6 xx 10^(-5) respectively. If a 0.01M solution of MX is added drop wise to a mixture containing A^(-) , B^(-) , C^(-)and D^(-) ions, then the one to be precipitated first will be : |
|
Answer» MA |
|
| 21183. |
What will be the molecular weight of NaCl determined experimentally following elevation in the boiling point or depression in freezing point method ? |
|
Answer» `LT 58.5` |
|
| 21184. |
Which one of the following is used as a catalyst in the conversion of Benzoic acid to Benzyl alcohol? |
|
Answer» NI |
|
| 21185. |
What is the range of electrical conductivity in insulators ? |
| Answer» SOLUTION :`10^(-20)-10^(-10)OHM^(-1)m^(-1)` | |
| 21186. |
What products do we get at cathode and anode during the electrolysis of molten and aqueous NaCl ? |
|
Answer» Solution :Moten NACL (i) Na is deposited at cathode. `Cl_(2)` is evolved at ANODE. Aqueous NaCl (II) `H_(2)` is evolved at cathode. (iii) `Cl_(2)` is evolved at anode. |
|
| 21187. |
Whichof thefollowingrotates the palne polarizedlight totwardsleft ? |
|
Answer» D(+) GLUCOSE |
|
| 21188. |
Which of the following equations does NOT represent Charles 's law for a given mass of gas at constant pressure? |
|
Answer» LOG V=log K+log T |
|
| 21189. |
Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA as shown in V-T diagram below: Work done by the gas during the entire cycle is : |
|
Answer» 600R (1-ln2) or `PV^2`=constant (K=2 for polytropic PROCESS) `:. W_(BC)=(nR)/(K-1)(T-T)=(2xxR)/(2-1)(1200-600)=1200R` Work done by gas during ENTIRE cycle `=W_(AB)+W_(BC)+W_(CA)` = - 1200 Rln2+1200 R+0 =1200 R(1-ln2) |
|
| 21190. |
Which of the following do not contain peroxide ions |
|
Answer» `PbO_(2)` |
|
| 21191. |
The standard reduction potentials of the metals A,B and C are 0.68, -2.50 and -0.50 V respectively. The order of their reducing power is : |
|
Answer» `A GT B gt C` |
|
| 21192. |
Which one of the following is employed as a tranquiliser ? |
|
Answer» Naproxen |
|
| 21193. |
Which is not true statement ? |
|
Answer» PROTEIN is polymer of `alpha`-amino acids |
|
| 21194. |
When tertiary butyl alcohol and 1-butanol are separately treated with a few drops of KMnO_(4), in one case only the purple colour disappears and a brown precipitate is formed. Which of the two alcohols gives the above reaction and what is that brown precipitate. |
|
Answer» Solution :1-butanol, being primary alcohol gets oxidised by dilute `KMnO_(4)`. The BROWN precipitate is DUE to the formation of manganese dioxide. `2KMNO_(4)+H_(2)Orarr 3KOH +underset("(brown precipitate)")underset("manganese dioxide")(2MnO_(2))+3(O)` `underset("1 - butanol")(CH_(3)-CH_(2)-CH_(2)-CH_(2)-OH)+overset(2(O))underset("room TEMP.")rarr underset("butanoic acid")(CH_(3)CH_(2)CH_(2)COOH)+H_(2)O` |
|
| 21195. |
Which of the following disease is caused by the deficiency of alpha-Tocopherol ? |
| Answer» Solution :Sterility | |
| 21196. |
Which is the last element in the series of the actinoids ? Write the electronic configuration of this element. Comment on the possible oxidation state of this element. |
|
Answer» Solution :Last ACTINOID is Lawrencium (Z = 103). Electronic configuration = `[RN]5f^(14)d^(1)7S^(2)` Possible oxidation state = +3. |
|
| 21197. |
Which member of the halogen family(X_2) does not show positive oxidation state (X_2^+)? |
|
Answer» Fluorine |
|
| 21198. |
The sex hormone which controls the development and maintanance of pregnancy is |
|
Answer» CORTISONE |
|
| 21199. |
Which ofthefollowingfactorsisof no significancefor roastingsulphideoresto the oxidesand notsubjectingthesulphideorestocarbonreductiondirectly ? |
|
Answer» ` CO_ 2`is morevolatile THN` CS _2 ` |
|
| 21200. |
The reduction potentials of four metals P,Q,R and Sare -2.90,+.34,+1.20 and -0.76 respectively. Reactivity dereases in the order. |
|
Answer» <P>`P GT Q gt R gt S` |
|